Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03MXK
|
|||
| Former ID |
DNCL003447
|
|||
| Drug Name |
AT-406
|
|||
| Synonyms |
1071992-99-8; SM 406; (5S,8S,10aR)-N-benzhydryl-5-((S)-2-(methylamino)propanamido)-3-(3-methylbutanoyl)-6-oxodecahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide; CHEMBL2158051; QCR-136; UNII-N65WC8PXDD; N65WC8PXDD; AT406 (SM-406, ARRY-334543); J-501625; (5S,8S,10aR)-N-(Diphenylmethyl)-5-[(N-methyl-L-alanyl)amino]-3-(3-methylbutanoyl)-6-oxodecahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide; AT-406/Debio-1143
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Squamous head and neck cell carcinom [ICD-11: 2D60.0; ICD-10: C77.0] | Phase 3 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [2] | ||
| Lymphoma [ICD-11: 2A80-2A86; ICD-9: 202.8, 208.9] | Phase 1/2 | [3] | ||
| Acute myeloid leukaemia [ICD-11: 2A60] | Phase 1 | [4], [5], [6] | ||
| Company |
Ascenta Therapeutics; Debiopharm
|
|||
| Structure |
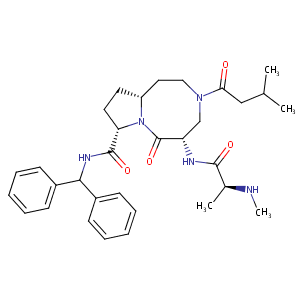 |
Download2D MOL |
||
| Formula |
C32H43N5O4
|
|||
| Canonical SMILES |
CC(C)CC(=O)N1CCC2CCC(N2C(=O)C(C1)NC(=O)C(C)NC)C(=O)NC(C3=CC=CC=C3)C4=CC=CC=C4
|
|||
| InChI |
1S/C32H43N5O4/c1-21(2)19-28(38)36-18-17-25-15-16-27(37(25)32(41)26(20-36)34-30(39)22(3)33-4)31(40)35-29(23-11-7-5-8-12-23)24-13-9-6-10-14-24/h5-14,21-22,25-27,29,33H,15-20H2,1-4H3,(H,34,39)(H,35,40)/t22-,25+,26-,27-/m0/s1
|
|||
| InChIKey |
LSXUTRRVVSPWDZ-MKKUMYSQSA-N
|
|||
| CAS Number |
CAS 1071992-99-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04459715) A Study of Debio 1143 (Xevinapant) in Combination With Platinum-Based Chemotherapy and Standard Fractionation Intensity-Modulated Radiotherapy in Participants With Locally Advanced Squamous Cell Carcinoma of the Head and Neck, Suitable for Definitive Chemoradiotherapy. U.S. National Institutes of Health. | |||
| REF 2 | Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50. | |||
| REF 3 | ClinicalTrials.gov (NCT02022098) Debio 1143-201 Dose-finding and Efficacy Phase I/II Trial. U.S. National Institutes of Health. | |||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7729). | |||
| REF 5 | A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011 Apr 28;54(8):2714-26. | |||
| REF 6 | J Clin Oncol 32:5s, 2014 (suppl; abstr 7029) | |||
| REF 7 | Debio 1143, an antagonist of multiple inhibitor-of-apoptosis proteins, activates apoptosis and enhances radiosensitization of non-small cell lung c... Am J Cancer Res. 2014 Nov 19;4(6):943-51. | |||
| REF 8 | Debio 1143, an antagonist of multiple inhibitor-of-apoptosis proteins, activates apoptosis and enhances radiosensitization of non-small cell lung cancer cells in vitro. Am J Cancer Res. 2014; 4(6): 943-951. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

