Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I8FI
|
|||
| Former ID |
DAP000080
|
|||
| Drug Name |
Isoproterenol
|
|||
| Synonyms |
isoproterenol; Isoprenaline; Isoprenalin; Norisodrine; Novodrin; Isopropydrin; Isopropylarterenol; Respifral; Assiprenol; Asiprenol; Bellasthman; Asmalar; Aludrine; Aludrin; N-Isopropylnoradrenaline; Bronkephrine; Neodrenal; Lomupren; Isonorene; Isopropyladrenaline; 7683-59-2; N-Isopropylnorepinephrine; Isopropylnorepinephrine; neo-Epinine; Isadrine; Saventrine; Isorenin; Isonorin; Proternol; Isopropylnoradrenaline; Isopropyl noradrenaline; Racemic isoprenaline; dl-Isadrine; Racemic isoproterenol; (+-)-Isoproterenol; Vapo-N-iso; Aerolone; Aleudrine; Dihydroxyphenylethanolisopropylamine; Euspiran; ISOPROP; Isadrin; Isoproterenolum; Isuprel; Isupren; Izadrin; Epinephrine isopropyl homolog; Isoprenaline hydrochloride; Isoproterenol Chloride; Isoproterenol [JAN]; Isuprel Mistometer; WIN 5162; D-Isoprenaline; D-Isopropylarterenol; D-Isoproterenol; DL-Isopropylnorepinephrine; Dl-Ipr; Dl-Isadrine; Dl-Isopropylnoradrenaline; Isoprenalina [INN-Spanish]; Isoprenaline (INN); Isoprenalinum [INN-Latin]; Isuprel (TN); L-Isopropylnoradrenaline; L-Isoproterenol; Medihaler-ISO; Neo-Epinine; Vapo-Iso; Alpha-(Isopropylaminomethyl)protocatechuyl alcohol; Alpha-(Isopropylaminomoethyl)protocatechuyl alcohol; D-N-Isopropylnorepinephrine; Dl-N-Isopropylnoradrenaline; Isopropylaminomethyl(3,4-dihydroxyphenyl)carbinol; Isopropylaminomethyl-3,4-dihydroxyphenyl carbinol; DL(+-)-Isoproterenol; N-Isopropyl-beta-dihydroxyphenyl-beta-hydroxyethylamine; (+)-Isoprenaline; (+)-Isoproterenol; (+-)-Isoprenaline; (-)-Isoproterenol hydrochloride; (S)-(+)-Isoproterenol; (S)-Isoprenaline; (S)-Isoproterenol; 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-(9CI); 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, (S)-(9CI); 1-(3,4-Dihydroxyphenyl)-2-(isopropylamino)ethanol; 1-(3,4-Dihydroxyphenyl)-2-isopropylaminoethanol; 3,4-Dihydroxy-alpha-(isopropylaminomethyl)-benzyl alcohol; 3,4-Dihydroxy-alpha-[(isopropylamino)methyl]benzyl alcohol; 3,4-Dihydroxy-alpha-((isopropylamino)methyl)benzyl alcohol; 4-(1-Hydroxy-2(isopropylamino)ethyl)-benzene 1,2-diol; 4-(1-Hydroxy-2-((1-methylethyl)amino)ethyl)-1,2-benzenediol; 4-{1-hydroxy-2-[(1-methylethyl)amino]ethyl}benzene-1,2-diol; AS1409
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Heart block [ICD-11: BC63] | Approved | [1] | |
| Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 1 | [2] | ||
| Therapeutic Class |
Cardiotonic Agents
|
|||
| Company |
Abbott Laboratories
|
|||
| Structure |
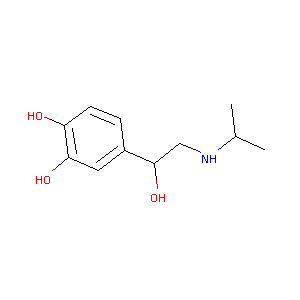 |
Download2D MOL |
||
| Formula |
C11H17NO3
|
|||
| Canonical SMILES |
CC(C)NCC(C1=CC(=C(C=C1)O)O)O
|
|||
| InChI |
1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3
|
|||
| InChIKey |
JWZZKOKVBUJMES-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 7683-59-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9268, 75322, 91788, 611308, 3133594, 5272313, 8152391, 10535368, 11336069, 11361308, 11363843, 11366405, 11368967, 11371528, 11373566, 11377129, 11462280, 11467125, 11468245, 11484778, 11486793, 11488877, 11490290, 11491839, 11494763, 11534308, 14748912, 24430805, 24715029, 26512260, 26752240, 29222898, 46507323, 47216837, 47291195, 47365254, 47440329, 47515375, 47810812, 47959817, 48334566, 48416134, 49892804, 49892806, 49892807, 50104946, 50111114, 53790889, 56313305, 56313643
|
|||
| ChEBI ID |
CHEBI:64317
|
|||
| ADReCS Drug ID | BADD_D01205 ; BADD_D01207 ; BADD_D01208 | |||
| SuperDrug ATC ID |
C01CA02; R03AB02; R03CB01
|
|||
| SuperDrug CAS ID |
cas=007683592
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 083346. | |||
| REF 2 | A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005. | |||
| REF 3 | Current therapeutic uses and potential of beta-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998 Feb;53(6):389-404. | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
| REF 5 | A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

