Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T95385
(Former ID: TTDI01746)
|
|||||
| Target Name |
Interleukin-12 beta (IL12B)
|
|||||
| Synonyms |
NKSF2; NK cell stimulatory factor chain 2; Interleukin12 subunit beta; Interleukin-12 subunit beta; IL12 subunit p40; IL-12B; IL-12 subunit p40; Cytotoxic lymphocyte maturation factor 40 kDa subunit; CLMF p40
Click to Show/Hide
|
|||||
| Gene Name |
IL12B
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Conduction disorder [ICD-11: BC63] | |||||
| Function |
Cytokine that can act as a growth factor for activated T and NK cells, enhance the lytic activity of NK/lymphokine-activated killer cells, and stimulate the production of IFN-gamma by resting PBMC.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: interleukin
|
|||||
| UniProt ID | ||||||
| Sequence |
MCHQQLVISWFSLVFLASPLVAIWELKKDVYVVELDWYPDAPGEMVVLTCDTPEEDGITW
TLDQSSEVLGSGKTLTIQVKEFGDAGQYTCHKGGEVLSHSLLLLHKKEDGIWSTDILKDQ KEPKNKTFLRCEAKNYSGRFTCWWLTTISTDLTFSVKSSRGSSDPQGVTCGAATLSAERV RGDNKEYEYSVECQEDSACPAAEESLPIEVMVDAVHKLKYENYTSSFFIRDIIKPDPPKN LQLKPLKNSRQVEVSWEYPDTWSTPHSYFSLTFCVQVQGKSKREKKDRVFTDKTSATVIC RKNASISVRAQDRYYSSSWSEWASVPCS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T84G6T | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Isoproterenol | Drug Info | Approved | Heart block | [2] | |
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | ABT-874 | Drug Info | Phase 3 | Plaque psoriasis | [3] | |
| 2 | EGEN-001 | Drug Info | Phase 2 | Ovarian cancer | [4] | |
| 3 | SFV IL-12 gene therapy | Drug Info | Phase 2 | Breast cancer | [5] | |
| 4 | Ad-IL-12 DNA therapeutic | Drug Info | Phase 1/2 | Melanoma | [6] | |
| 5 | NHS-IL 12 | Drug Info | Phase 1 | Solid tumour/cancer | [7] | |
| 6 | PF-07261271 | Drug Info | Phase 1 | Inflammatory bowel disease | [8] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | Isoproterenol | Drug Info | [1] | |||
| 2 | ABT-874 | Drug Info | [9] | |||
| 3 | EGEN-001 | Drug Info | [10] | |||
| 4 | SFV IL-12 gene therapy | Drug Info | [11] | |||
| 5 | Ad-IL-12 DNA therapeutic | Drug Info | [12] | |||
| 6 | NHS-IL 12 | Drug Info | [13] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 5-Mercapto-2-nitro-benzoic acid | Ligand Info | |||||
| Structure Description | THE P40 DOMAIN OF HUMAN INTERLEUKIN-12 | PDB:1F42 | ||||
| Method | X-ray diffraction | Resolution | 2.50 Å | Mutation | No | [15] |
| PDB Sequence |
IWELKKDVYV
10 VELDWYPDAP20 GEMVVLTCDT30 PEEDGITWTL40 DQSSEVLGSG50 KTLTIQVKEF 60 GDAGQYTCHK70 GGEVLSHSLL80 LLHKKEDGIW90 STDILKDQKE100 PKNKTFLRCE 110 AKNYSGRFTC120 WWLTTISTDL130 TFSVKSSRGG145 VTCGAATLSA155 ERVRGDNKEY 165 EYSVECQEDS175 ACPAAEESLP185 IEVMVDAVHK195 LKYENYTSSF205 FIRDIIKPDP 215 PKNLQLKPLK225 NSRQVEVSWE235 YPDTWSTPHS245 YFSLTFCVQV255 QGKSKRRVFT 269 DKTSATVICR279 KNASISVRAQ289 DRYYSSSWSE299 WASVPCS
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|


| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
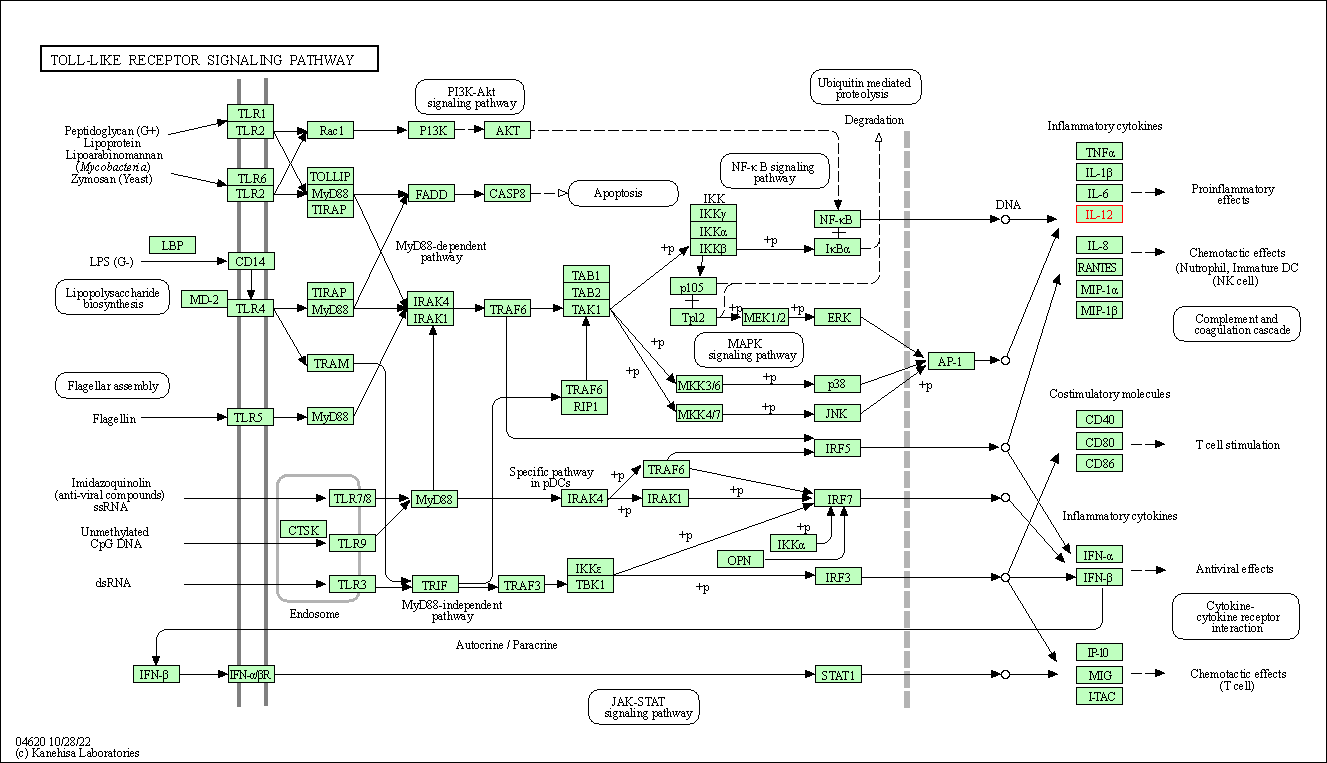
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
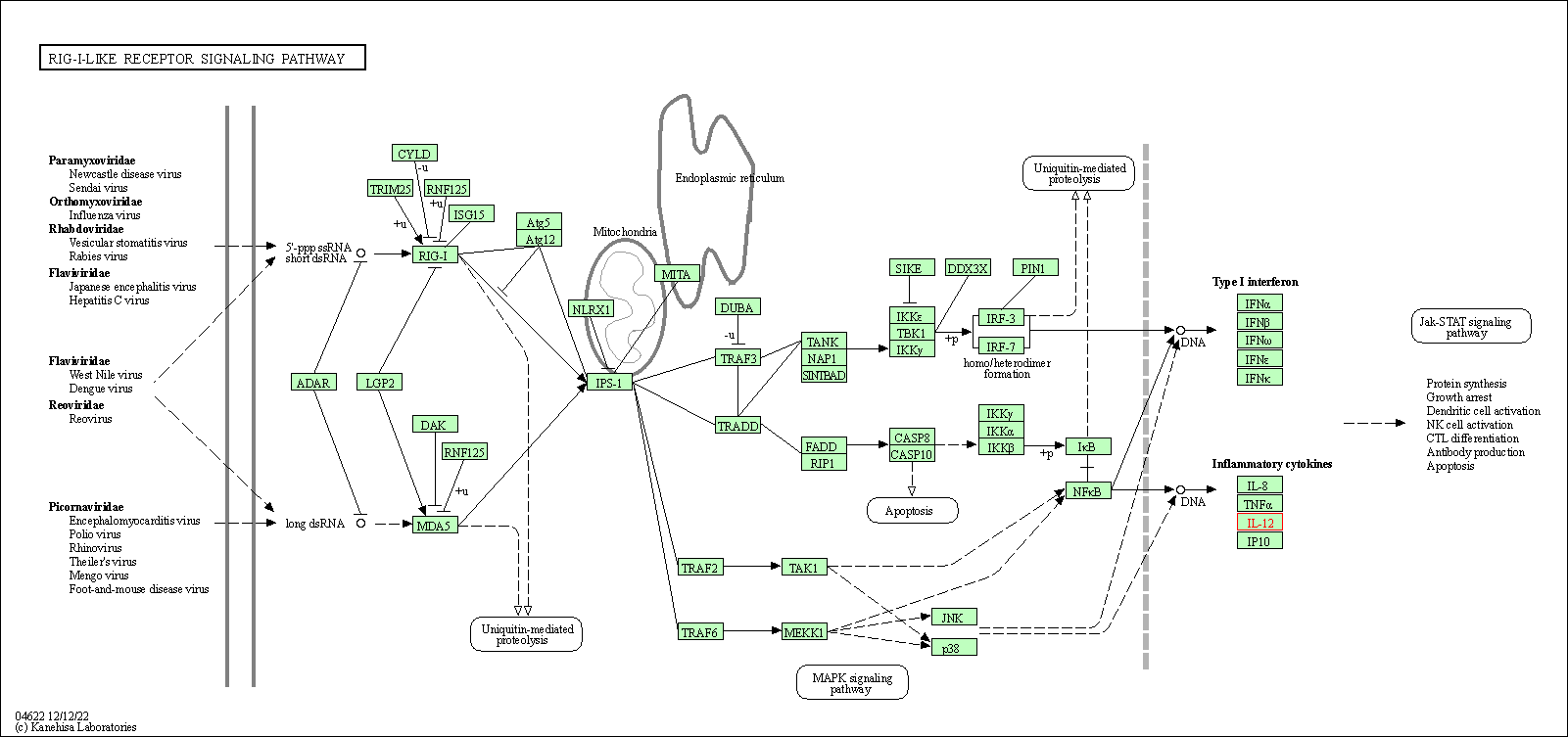
| RIG-I-like receptor signaling pathway | hsa04622 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
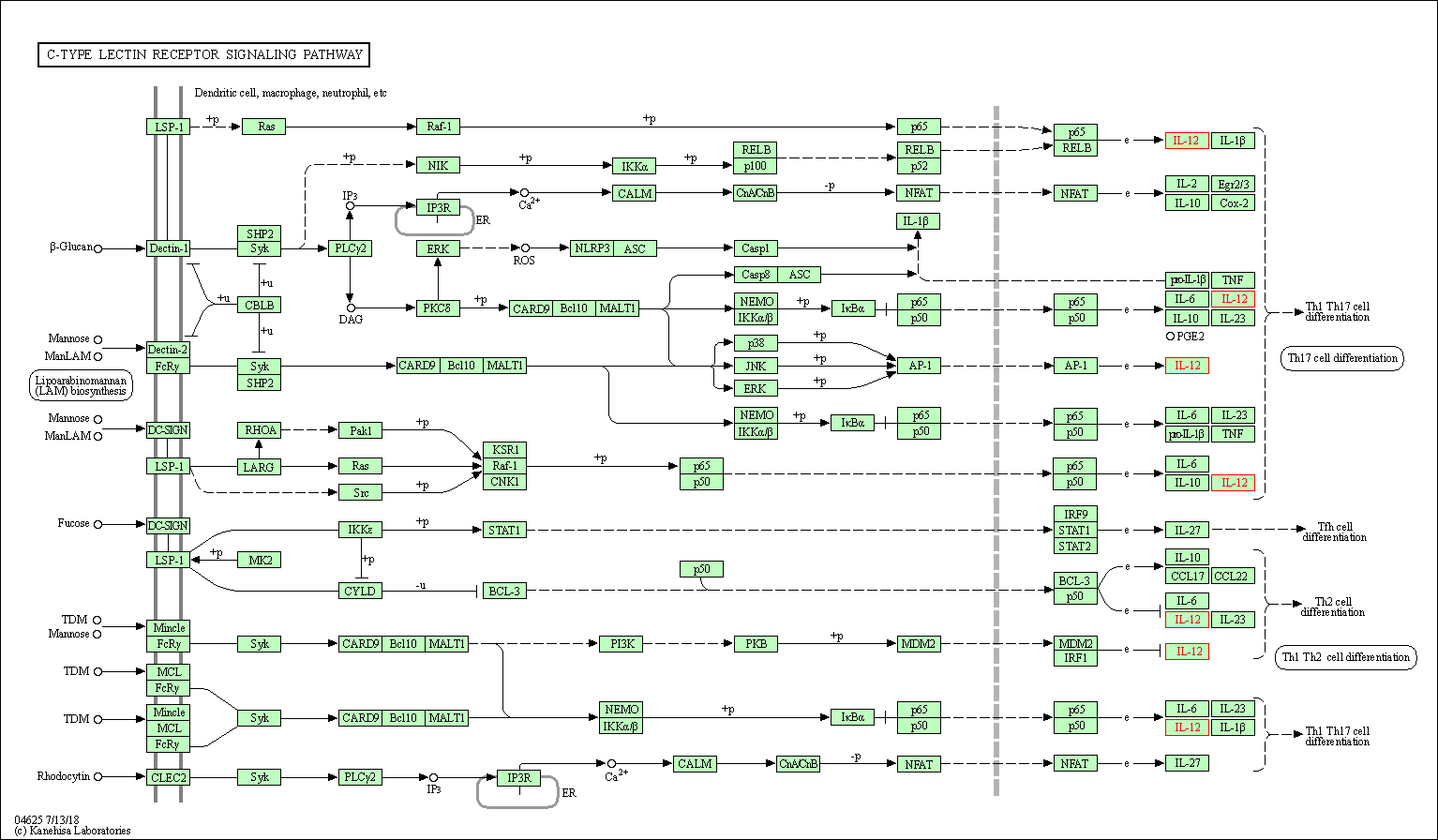
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
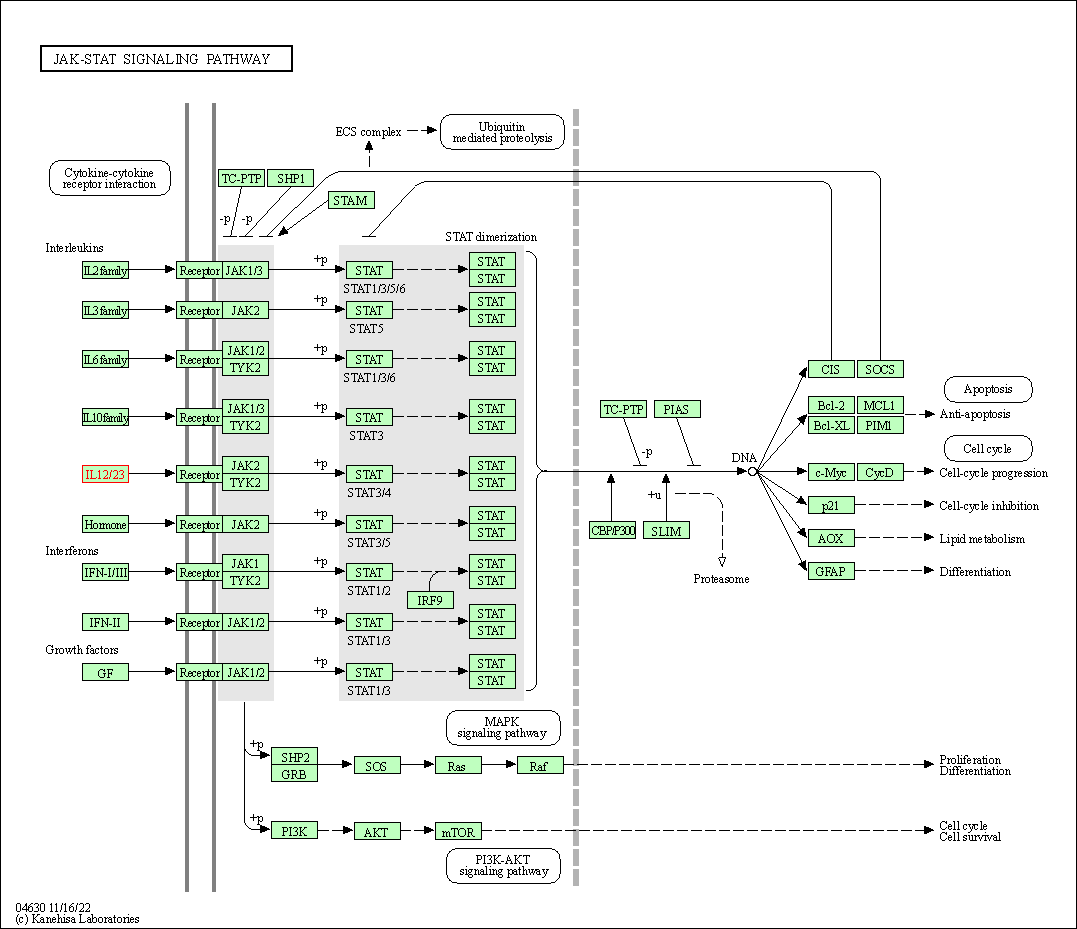
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Th1 and Th2 cell differentiation | hsa04658 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 7 | Degree centrality | 7.52E-04 | Betweenness centrality | 1.01E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.99E-01 | Radiality | 1.35E+01 | Clustering coefficient | 4.29E-01 |
| Neighborhood connectivity | 2.19E+01 | Topological coefficient | 2.63E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 19 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | Toll-like receptor signaling pathway | |||||
| 3 | RIG-I-like receptor signaling pathway | |||||
| 4 | Jak-STAT signaling pathway | |||||
| 5 | Type I diabetes mellitus | |||||
| 6 | Pertussis | |||||
| 7 | Legionellosis | |||||
| 8 | Leishmaniasis | |||||
| 9 | Chagas disease (American trypanosomiasis) | |||||
| 10 | African trypanosomiasis | |||||
| 11 | Malaria | |||||
| 12 | Toxoplasmosis | |||||
| 13 | Amoebiasis | |||||
| 14 | Tuberculosis | |||||
| 15 | Measles | |||||
| 16 | Influenza A | |||||
| 17 | Herpes simplex infection | |||||
| 18 | Inflammatory bowel disease (IBD) | |||||
| 19 | Allograft rejection | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | Toll-like receptor signaling pathway | |||||
| 2 | Aryl Hydrocarbon Receptor Pathway | |||||
| 3 | Allograft Rejection | |||||
| 4 | Regulation of toll-like receptor signaling pathway | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1998-2005. | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 083346. | |||||
| REF 3 | Emerging drugs for rheumatoid arthritis. Expert Opin Emerg Drugs. 2008 Mar;13(1):175-96. | |||||
| REF 4 | ClinicalTrials.gov (NCT01118052) EGEN-001 in Treating Patients With Persistent or Recurrent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer. U.S. National Institutes ofHealth. | |||||
| REF 5 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036273) | |||||
| REF 6 | Clinical pipeline report, company report or official report of ZIOPHARM. | |||||
| REF 7 | ClinicalTrials.gov (NCT01417546) NHS-IL12 for Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT05536440) A PHASE 1, RANDOMIZED, DOUBLE-BLIND, SPONSOR OPEN, PLACEBO CONTROLLED, DOSE ESCALATING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF SINGLE INTRAVENOUS AND MULTIPLE SUBCUTANEOUS AND INTRAVENOUS DOSES OF PF-07261271 IN HEALTHY PARTICIPANTS. U.S.National Institutes of Health. | |||||
| REF 9 | Briakinumab for the treatment of plaque psoriasis.BioDrugs.2012 Feb 1;26(1):9-20. | |||||
| REF 10 | National Cancer Institute Drug Dictionary (drug id 454593). | |||||
| REF 11 | Clinical pipeline report, company report or official report of Trademarkia. | |||||
| REF 12 | National Cancer Institute Drug Dictionary (drug id 710508). | |||||
| REF 13 | The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget. 2014 Apr 15;5(7):1869-84. | |||||
| REF 14 | Clinical pipeline report, company report or official report of Pfizer | |||||
| REF 15 | Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000 Jul 17;19(14):3530-41. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

