Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0NG7O
|
|||
| Former ID |
DAP000077
|
|||
| Drug Name |
Zolmitriptan
|
|||
| Synonyms |
AscoTop; Flezol; Zolmitriptane; Zolmitriptanum; Zomig; Zomigon; Zolmitriptan RapidFilm; Zolmitriptan [USAN]; Zomig Nasal Spray; Zomig ZMT; AscoTop (TN); BW-311C90; KS-5072; Zomig (TN); Zomig-ZMT; Zomigon (TN); Zomigoro (TN); Zomig, Zomigon, AscoTopand, Zomigoro, Zolmitriptan; (4S)-4-({3-[2-(dimethylamino)ethyl]-1H-indol-5-yl}methyl)-1,3-oxazolidin-2-one; (4S)-4-[[3-(2-dimethylaminoethyl)-1H-indol-5-yl]methyl]-1,3-oxazolidin-2-one; (S)-4-((3-(2-(Dimethylamino)ethyl)-1H-indol-5-yl)methyl)-2-oxazolidinone; (S)-4-((3-(2-(Dimethylamino)ethyl)indol-5-yl)methyl)-2-oxazolidinone; (S)-4-[3-(2-Dimethylamino-ethyl)-1H-indol-5-ylmethyl]-oxazolidin-2-one; (S)-4-[[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone; 4-((3-(2-(Dimethylamino)ethyl)-1H-indol-5-yl)methyl)-2-oxazolidinone; 4-[[3-(2-dimethylaminoethyl)-1H-indol-5-yl]methyl]oxazolidin-2-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Approved | [1], [2] | |
| Therapeutic Class |
Antimigraine Agents
|
|||
| Company |
AstraZeneca
|
|||
| Structure |
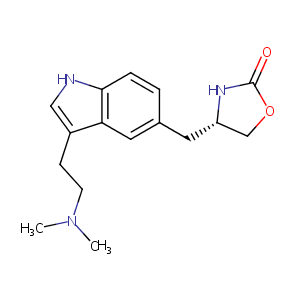 |
Download2D MOL |
||
| Formula |
C16H21N3O2
|
|||
| Canonical SMILES |
CN(C)CCC1=CNC2=C1C=C(C=C2)CC3COC(=O)N3
|
|||
| InChI |
1S/C16H21N3O2/c1-19(2)6-5-12-9-17-15-4-3-11(8-14(12)15)7-13-10-21-16(20)18-13/h3-4,8-9,13,17H,5-7,10H2,1-2H3,(H,18,20)/t13-/m0/s1
|
|||
| InChIKey |
ULSDMUVEXKOYBU-ZDUSSCGKSA-N
|
|||
| CAS Number |
CAS 139264-17-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9427, 7847481, 7980917, 8187100, 11484392, 11488544, 11528684, 12014671, 14897880, 14922369, 26612872, 26680025, 26719749, 43118195, 46386880, 46386934, 46506452, 46530532, 49681573, 50730852, 57314155, 91011737, 92124588, 92308124, 92308640, 93166198, 96099961, 103346351, 103941674, 104179250, 104253285, 104321827, 117664449, 118855343, 124636835, 124757406, 124801240, 125164210, 126656630, 126667001, 129386325, 135017989, 135651366, 135692210, 135693782, 136974858, 137171693, 142742126, 144076376, 144205008
|
|||
| ChEBI ID |
CHEBI:10124
|
|||
| ADReCS Drug ID | BADD_D02393 | |||
| SuperDrug ATC ID |
N02CC03
|
|||
| SuperDrug CAS ID |
cas=139264178
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 1B receptor (HTR1B) | Target Info | Modulator | [3] |
| 5-HT 1D receptor (HTR1D) | Target Info | Modulator | [3] | |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT1 type receptor mediated signaling pathway | ||||
| Reactome | Serotonin receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 60). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 020768. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

