Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T28330
(Former ID: TTDC00082)
|
|||||
| Target Name |
Cholecystokinin receptor type A (CCKAR)
|
|||||
| Synonyms |
CCKAR; CCK-AR; CCK-A receptor
Click to Show/Hide
|
|||||
| Gene Name |
CCKAR
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Influenza [ICD-11: 1E30-1E32] | |||||
| 2 | Pancreatic malfunction [ICD-11: DC30-DC3Z] | |||||
| Function |
Receptor forcholecystokinin. Mediates pancreatic growth and enzyme secretion, smooth muscle contraction of the gall bladder and stomach. Has a 1000-fold higher affinity for CCK rather than for gastrin. It modulates feeding and dopamine-induced behavior in the central and peripheral nervous system. This receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MDVVDSLLVNGSNITPPCELGLENETLFCLDQPRPSKEWQPAVQILLYSLIFLLSVLGNT
LVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYF MGTSVSVSTFNLVAISLERYGAICKPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYS NLVPFTKNNNQTANMCRFLLPNDVMQQSWHTFLLLILFLIPGIVMMVAYGLISLELYQGI KFEASQKKSAKERKPSTTSSGKYEDSDGCYLQKTRPPRKLELRQLSTGSSSRANRIRSNS SAANLMAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTASAERRLSGTPISFILLLSY TSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGARGEVGEEEEGGTTGASLSRFSYSH MSASVPPQ Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 2 Clinical Trial Drugs | + | ||||
| 1 | Dexloxiglumide | Drug Info | Phase 2 | Pancreatic malfunction | [2] | |
| 2 | proglumide | Drug Info | Phase 2 | Influenza virus infection | [3] | |
| Discontinued Drug(s) | [+] 15 Discontinued Drugs | + | ||||
| 1 | CE-326597 | Drug Info | Discontinued in Phase 2 | Obesity | [4] | |
| 2 | GI 181771 | Drug Info | Discontinued in Phase 2 | Obesity | [5] | |
| 3 | Lintitript | Drug Info | Discontinued in Phase 2 | Obesity | [6], [7] | |
| 4 | Pranazepide | Drug Info | Discontinued in Phase 2 | Pancreatic malfunction | [8], [9] | |
| 5 | Tarazepide | Drug Info | Discontinued in Phase 2 | Gastrointestinal disease | [10] | |
| 6 | SR-146131 | Drug Info | Discontinued in Phase 1 | Obesity | [11], [12] | |
| 7 | SSR-125180 | Drug Info | Discontinued in Phase 1 | Obesity | [13] | |
| 8 | T-0632 | Drug Info | Discontinued in Phase 1 | Pancreatic malfunction | [14], [15] | |
| 9 | UCL-2000; butabindide | Drug Info | Discontinued in Phase 1 | Obesity | [16] | |
| 10 | A-70276 | Drug Info | Terminated | Gastrointestinal disease | [19] | |
| 11 | A-71623 | Drug Info | Terminated | Obesity | [20], [21] | |
| 12 | FR-208419 | Drug Info | Terminated | Dyspepsia | [22] | |
| 13 | GI-248573 | Drug Info | Terminated | Bladder disease | [23] | |
| 14 | Lorglumide | Drug Info | Terminated | Pancreatic malfunction | [24], [25] | |
| 15 | SR-27950 | Drug Info | Terminated | Eating disorder | [26] | |
| Preclinical Drug(s) | [+] 6 Preclinical Drugs | + | ||||
| 1 | A-71378 | Drug Info | Preclinical | Obesity | [17], [18] | |
| 2 | A-74498 | Drug Info | Preclinical | Obesity | [18] | |
| 3 | AR-R-1589 | Drug Info | Preclinical | Eating disorder | [18] | |
| 4 | FPL-14294 | Drug Info | Preclinical | Obesity | [18] | |
| 5 | GG-8573 | Drug Info | Preclinical | Obesity | [18] | |
| 6 | PD-170292 | Drug Info | Preclinical | Obesity | [18] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Inhibitor | [+] 51 Inhibitor drugs | + | ||||
| 1 | Dexloxiglumide | Drug Info | [27] | |||
| 2 | Pranazepide | Drug Info | [32] | |||
| 3 | CR-1795 | Drug Info | [41] | |||
| 4 | IQM-95333 | Drug Info | [43] | |||
| 5 | PD-135666 | Drug Info | [45] | |||
| 6 | SNF-9007 | Drug Info | [46] | |||
| 7 | 1-(3,3-Diphenyl-allyl)-3-m-tolyl-urea | Drug Info | [48] | |||
| 8 | 1-(4-Chloro-phenyl)-3-(3,3-diphenyl-allyl)-urea | Drug Info | [48] | |||
| 9 | 1-(4-Chloro-phenyl)-3-(3-pentyl-oct-2-enyl)-urea | Drug Info | [48] | |||
| 10 | 3,4-Dichloro-N-(3,3-diphenyl-allyl)-benzamide | Drug Info | [48] | |||
| 11 | Asp-Tyr(OSO3H)-Met-Gly-Trp-Met-Asp-Phe | Drug Info | [50] | |||
| 12 | Boc-Asp-Tyr(So3-)-Nle-Gly-Trp-Asp-Phe-NH2 | Drug Info | [53] | |||
| 13 | Boc-cyclo-(Glu-Tyr-Nle-D-Lys)-Trp-Nle-Asp-Phe-NH2 | Drug Info | [54] | |||
| 14 | Boc-D-Glu-Tyr(SO3H)-Nle-D-Lys-Trp-Nle-Asp-Phe-NH2 | Drug Info | [54] | |||
| 15 | Boc-D-Glu-Tyr(SO3H)-Nle-D-Nle-Trp-Nle-Asp-Phe-NH2 | Drug Info | [54] | |||
| 16 | Boc-Glu-Tyr(SO3H)-Nle-D-Lys-Trp-Nle-Asp-Phe-NH2 | Drug Info | [54] | |||
| 17 | Boc-Tyr(SO3H)-Nle-Gly-Trp-Nle-Asp-Phe-NH2 | Drug Info | [54] | |||
| 18 | CR-2345 | Drug Info | [41] | |||
| 19 | FR-175985 | Drug Info | [32] | |||
| 20 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle-Asp-Phe-H | Drug Info | [60] | |||
| 21 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-Nle-D-Trp-H | Drug Info | [61] | |||
| 22 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-Nle-Trp-Boc | Drug Info | [61] | |||
| 23 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Trp-D-Nle-D-Asp-D-Phe-H | Drug Info | [60] | |||
| 24 | L-708474 | Drug Info | [66] | |||
| 25 | L-740093 | Drug Info | [68] | |||
| 26 | PD-135118 | Drug Info | [45] | |||
| 27 | PD-136621 | Drug Info | [45] | |||
| 28 | PD-137337 | Drug Info | [45] | |||
| 29 | PD-137342 | Drug Info | [45] | |||
| 30 | PD-138915 | Drug Info | [45] | |||
| 31 | PD-138916 | Drug Info | [45] | |||
| 32 | PD-140547 | Drug Info | [45] | |||
| 33 | PD-140548 | Drug Info | [45] | |||
| 34 | PD-140723 | Drug Info | [45] | |||
| 35 | Tetragastrin | Drug Info | [69] | |||
| 36 | Tyr-D-Ala-Gly-D-Trp-Nle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 37 | Tyr-D-Ala-Gly-D-Trp-NMeNle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 38 | Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-NMeNle-D-Trp-Boc | Drug Info | [61] | |||
| 39 | Tyr-D-Ala-Gly-Trp-Nle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 40 | Tyr-D-Ala-Gly-Trp-NMeNle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 41 | Tyr-D-Nle-Gly-D-Trp-NMeNle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 42 | Tyr-D-Nle-Gly-Trp-Nle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 43 | Tyr-D-Phe-Gly-D-Trp-Nle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 44 | Tyr-D-Phe-Gly-D-Trp-NMeNle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 45 | Tyr-D-Phe-Gly-Trp-Nle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 46 | Tyr-D-Phe-Gly-Trp-NMeNle-Asp-Phe-NH2 | Drug Info | [46] | |||
| 47 | VL-0395 | Drug Info | [71] | |||
| 48 | VL-0494 | Drug Info | [72] | |||
| 49 | VL-0699 | Drug Info | [73] | |||
| 50 | VL-1499 | Drug Info | [71] | |||
| 51 | VL-2799 | Drug Info | [74] | |||
| Antagonist | [+] 18 Antagonist drugs | + | ||||
| 1 | proglumide | Drug Info | [1] | |||
| 2 | Lintitript | Drug Info | [30], [31] | |||
| 3 | Tarazepide | Drug Info | [33], [34], [35] | |||
| 4 | T-0632 | Drug Info | [38] | |||
| 5 | Lorglumide | Drug Info | [44] | |||
| 6 | 2-NAP | Drug Info | [49] | |||
| 7 | Asperlicin | Drug Info | [51] | |||
| 8 | Bis(31/31')[[Cys(31), Nva(34)]NPY(27-36)-NH(2)] | Drug Info | [52] | |||
| 9 | CI-1015 | Drug Info | [57] | |||
| 10 | Devazepide | Drug Info | [51] | |||
| 11 | IQM-97423 | Drug Info | [62] | |||
| 12 | JNJ-17156516 | Drug Info | [64] | |||
| 13 | KSG-504 | Drug Info | [65] | |||
| 14 | L-736380 | Drug Info | [67] | |||
| 15 | PD-135158 | Drug Info | [56] | |||
| 16 | SC-50998 | Drug Info | [47] | |||
| 17 | TP-680 | Drug Info | [70] | |||
| 18 | [3H]devazepide | Drug Info | [75] | |||
| Agonist | [+] 16 Agonist drugs | + | ||||
| 1 | CE-326597 | Drug Info | [28] | |||
| 2 | GI 181771 | Drug Info | [29] | |||
| 3 | SR-146131 | Drug Info | [36] | |||
| 4 | SSR-125180 | Drug Info | [37] | |||
| 5 | UCL-2000; butabindide | Drug Info | [18] | |||
| 6 | A-71378 | Drug Info | [18] | |||
| 7 | A-74498 | Drug Info | [18] | |||
| 8 | AR-R-1589 | Drug Info | [18] | |||
| 9 | FPL-14294 | Drug Info | [18] | |||
| 10 | GG-8573 | Drug Info | [18] | |||
| 11 | PD-170292 | Drug Info | [18] | |||
| 12 | CCK-33 | Drug Info | [55] | |||
| 13 | CCK-8 | Drug Info | [56] | |||
| 14 | Glaxo-11p | Drug Info | [58] | |||
| 15 | GW-5823 | Drug Info | [59] | |||
| 16 | JMV180 | Drug Info | [63] | |||
| Modulator | [+] 5 Modulator drugs | + | ||||
| 1 | A-70276 | Drug Info | [39] | |||
| 2 | A-71623 | Drug Info | [40] | |||
| 3 | FR-208419 | Drug Info | [42] | |||
| 4 | GI-248573 | Drug Info | [37] | |||
| 5 | SR-27950 | Drug Info | [47] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: SR-146131 | Ligand Info | |||||
| Structure Description | Structural insights into human brain gut peptide cholecystokinin receptors | PDB:7XOV | ||||
| Method | Electron microscopy | Resolution | 3.00 Å | Mutation | Yes | [76] |
| PDB Sequence |
AVQILLYSLI
51 FLLSVLGNTL61 VITVLIRNKR71 MRTVTNIFLL81 SLAVSDLMLC91 LFCMPFNLIP 101 NLLKDFIFGS111 AVCKTTTYFM121 GTSVSVSTFN131 LVAISLERYG141 AICKPLQSRV 151 WQTKSHALKV161 IAATWCLSFT171 IMTPYPIYSN181 LVPFTKNNNQ191 TANMCRFLLP 201 NDVMQQSWHT211 FLLLILFLIP221 GIVMMVAYGL231 ISLELYQGIK241 FEASAANLMA 307 KKRVIRMLIV317 IVVLFFLCWM327 PIFSANAWRA337 YDTASAERRL347 SGTPISFILL 357 LSYTSSCVNP367 IIYCFMNKRF377 RLGFMATF
|
|||||
|
|
LEU90
4.406
CYS94
3.779
ASN98
3.299
LEU99
3.247
ASN102
3.861
THR118
3.571
MET121
3.494
GLY122
2.919
THR123
4.840
VAL125
4.041
SER126
4.911
MET173
3.138
TYR176
2.432
TYR179
3.580
ARG197
3.253
LEU213
4.611
|
|||||
| Ligand Name: Devazepide | Ligand Info | |||||
| Structure Description | Crystal structure of the cholecystokinin receptor CCKAR in complex with devazepide | PDB:7F8Y | ||||
| Method | X-ray diffraction | Resolution | 2.50 Å | Mutation | Yes | [77] |
| PDB Sequence |
KEWQPAVQIL
46 LYSLIFLLSV56 LGNTLVITVL66 IRNKRMRTVT76 NIFLLSLAVS86 NLMLCLFCMP 96 FNLIPNLLKD106 FIFGSAVCKT116 TTYFMGTSVS126 VSTWNLVAIS136 LERYGAICKP 146 LQSRVWQTKS156 HALKVIAATW166 CLSFTIMTPY176 PIYSNLVPFT186 KNNNQTANMC 196 RFLLPNDVMQ206 QSWHTFLLLI216 LFLIPGIVMM226 VAYGLISLEL236 YQGINIFEML 1246 RIDEGLRLKI1256 YKDTEGYYTI1266 GIGHLLTKSP1276 SLNAAKSELD1286 KAIGRNTNGV 1296 ITKDEAEKLF1306 NQDVDAAVRG1316 ILRNAKLKPV1326 YDSLDAVRRA1336 ALINMVFQMG 1346 ETGVAGFTNS1356 LRMLQQKRWD1366 EAAVNLAKSR1376 WYNQTPNRAK1386 RVITTFRTGT 1396 WDAYAANLMA307 KKRVIRMLIV317 IVVLFFLCWM327 PIFSANAWRA337 YDTASAERRL 347 SGTPISFILL357 LSYTSSCVNP367 IIYCFMNK
|
|||||
|
|
PHE97
4.869
ASN98
3.041
THR117
3.381
THR118
3.437
MET121
3.584
GLY122
4.202
TYR176
3.839
CYS196
3.245
ARG197
4.097
PHE198
4.291
ILE329
4.149
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
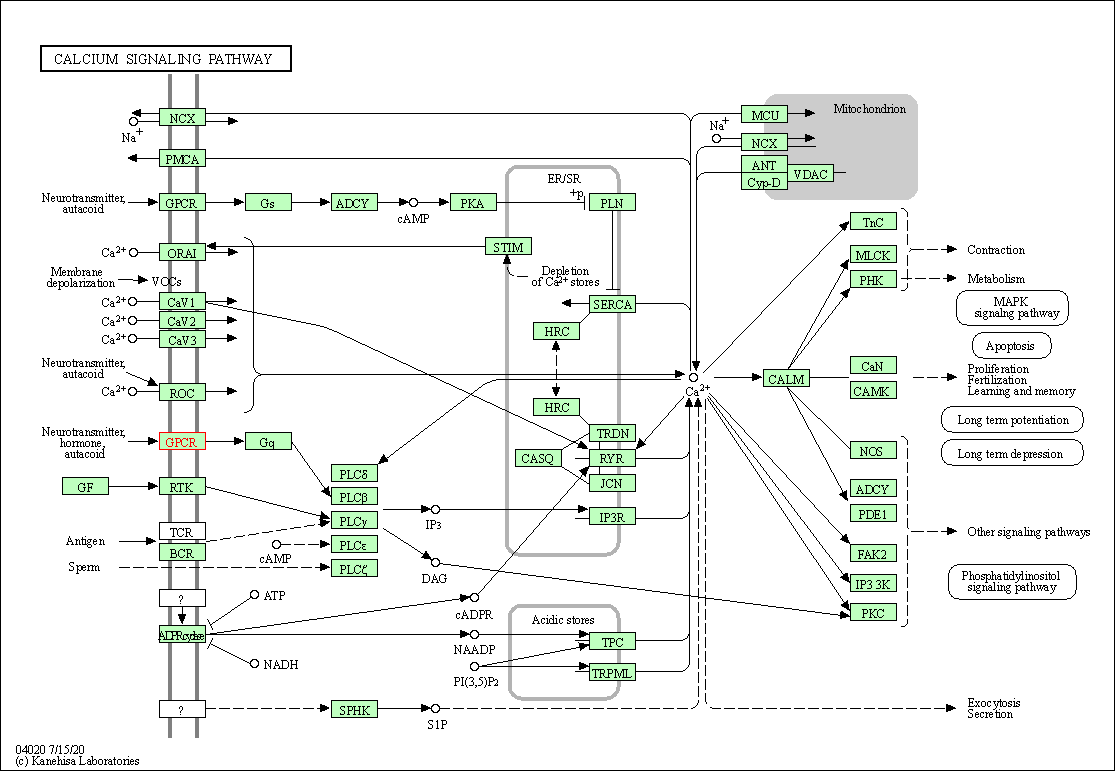
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
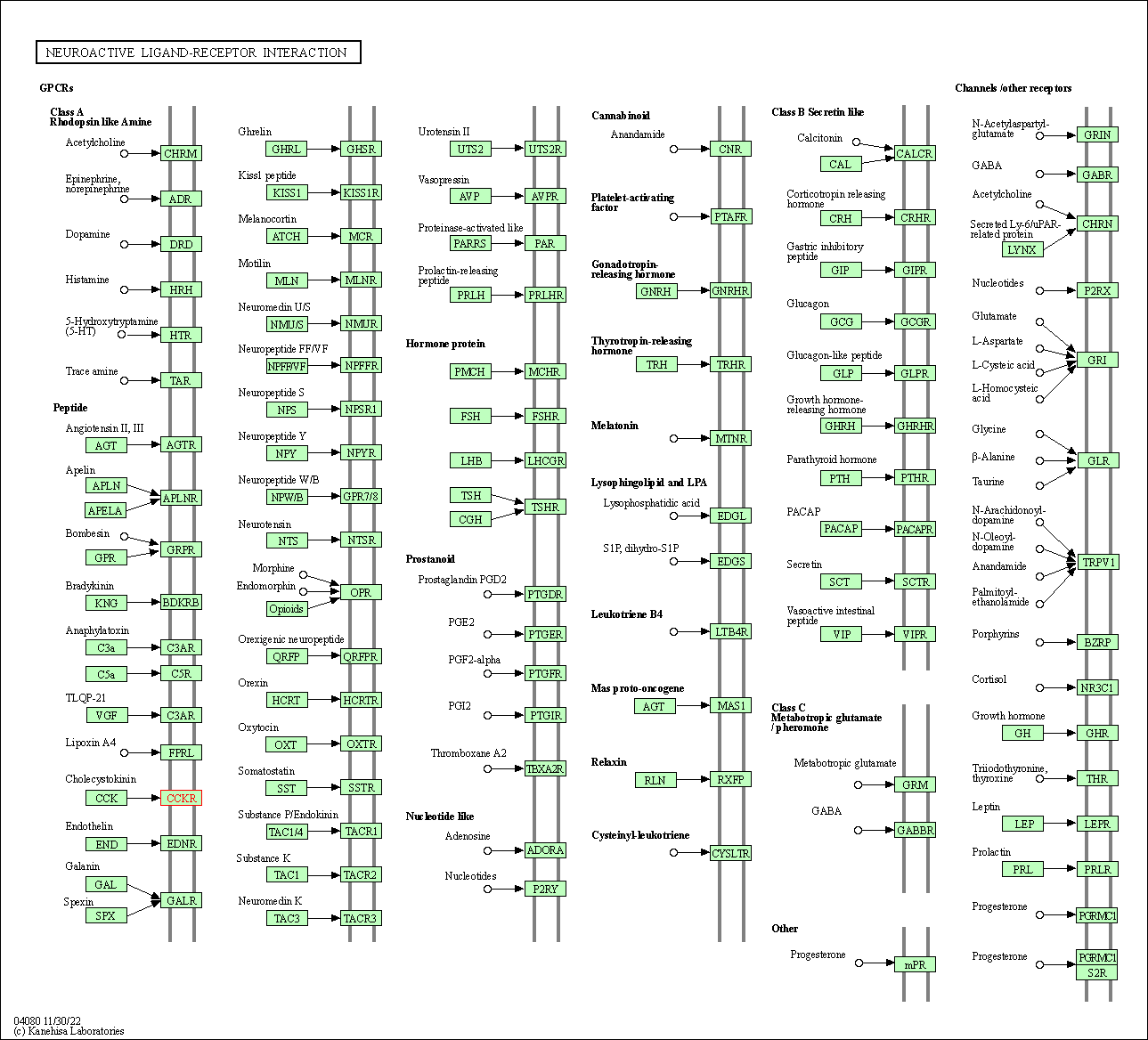
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
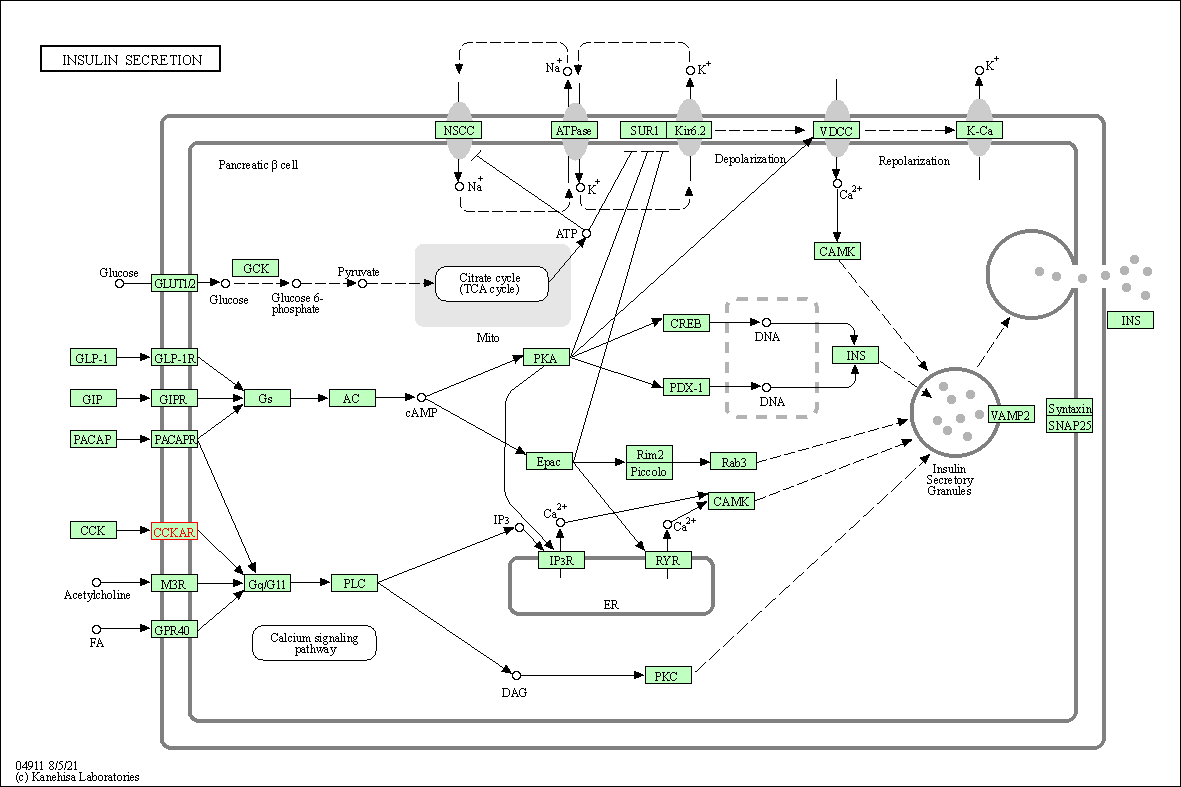
| Insulin secretion | hsa04911 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
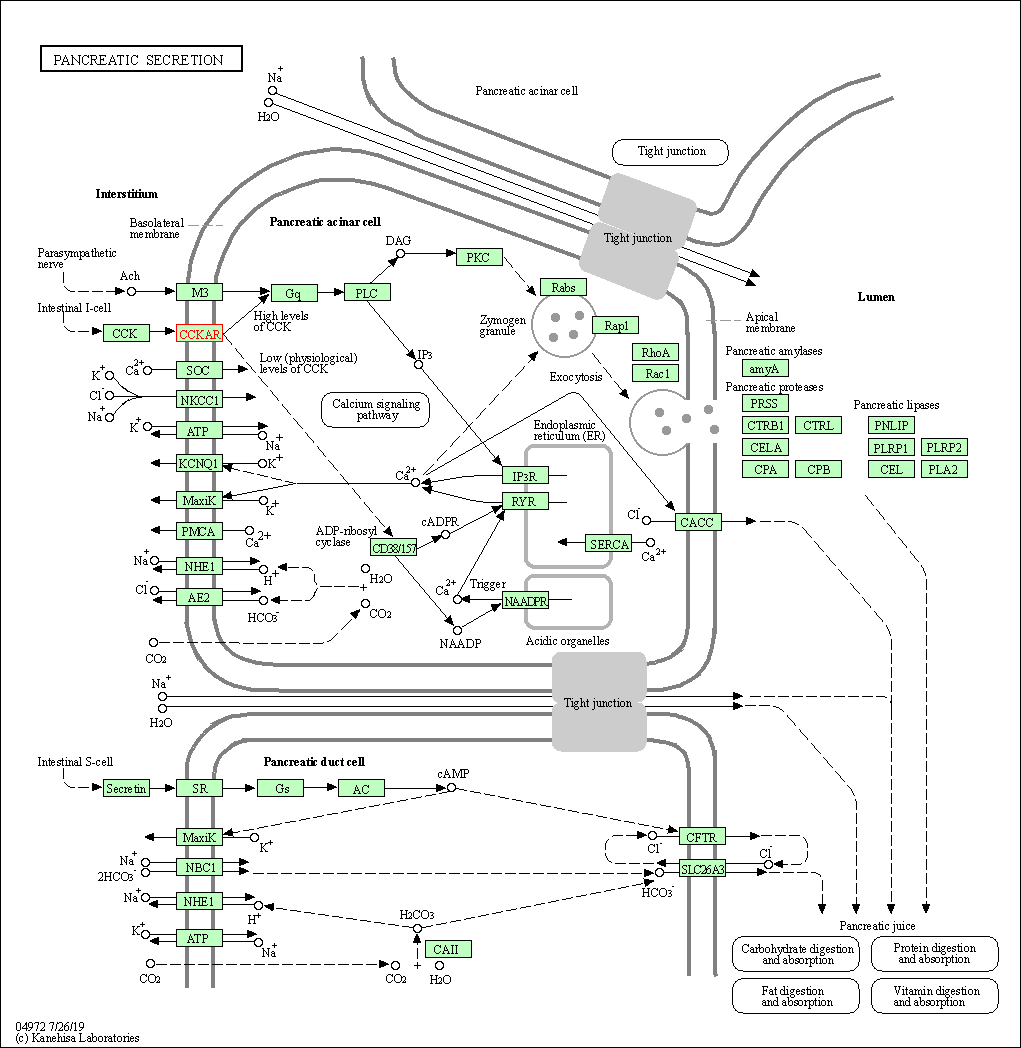
| Pancreatic secretion | hsa04972 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Degree | 1 | Degree centrality | 1.07E-04 | Betweenness centrality | 0.00E+00 |
|---|---|---|---|---|---|
| Closeness centrality | 1.56E-01 | Radiality | 1.22E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 7.00E+00 | Topological coefficient | 1.00E+00 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Calcium signaling pathway | |||||
| 2 | Neuroactive ligand-receptor interaction | |||||
| 3 | Insulin secretion | |||||
| 4 | Pancreatic secretion | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Peptide ligand-binding receptors | |||||
| 2 | G alpha (q) signalling events | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | GPCRs, Class A Rhodopsin-like | |||||
| 2 | Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| 3 | Peptide GPCRs | |||||
| 4 | GPCR ligand binding | |||||
| 5 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Pharmacological properties of lorglumide as a member of a new class of cholecystokinin antagonists. Arzneimittelforschung. 1987 Nov;37(11):1265-8. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | ClinicalTrials.gov (NCT00542009) A Trial In Diabetic Patients To Assess Effect Of CE-326,597 On Glucose Control And Body Weight. U.S. National Institutes of Health. | |||||
| REF 5 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013294) | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 890). | |||||
| REF 7 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004912) | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 874). | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003071) | |||||
| REF 10 | Cholecystokinin receptor-1 mediates the inhibitory effects of exogenous cholecystokinin octapeptide on cellular morphine dependence. BMC Neurosci. 2012 Jun 8;13:63. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 868). | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010439) | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015967) | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 884). | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007781) | |||||
| REF 16 | Clinical pipeline report, company report or official report of University College London. | |||||
| REF 17 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 894). | |||||
| REF 18 | Emerging drugs for obesity: linking novel biological mechanisms to pharmaceutical pipelines. Expert Opin Emerg Drugs. 2005 Aug;10(3):643-60. | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003433) | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 858). | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003438) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010485) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008844) | |||||
| REF 24 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 891). | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000580) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001260) | |||||
| REF 27 | Full agonists of CCK8 containing a nonhydrolyzable sulfated tyrosine residue. J Med Chem. 1989 Feb;32(2):445-9. | |||||
| REF 28 | Obesity Pharmacotherapy: Current Perspectives and Future Directions. Curr Cardiol Rev. 2013 February; 9(1): 33-54. | |||||
| REF 29 | Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2003 Jul-Aug;25(6):483-506. | |||||
| REF 30 | A cholecystokinin-1 receptor agonist (CCK-8) mediates increased permeability of brain barriers to leptin. Br J Pharmacol. 2008 Jul;154(5):1009-15. | |||||
| REF 31 | A novel role for cholecystokinin: regulation of mesenteric vascular resistance. Regul Pept. 2004 Sep 15;121(1-3):145-53. | |||||
| REF 32 | Dual CCK-A and -B receptor antagonists (I) C9-methyl-1,4-benzodiazepines, Bioorg. Med. Chem. Lett. 7(2):169-174 (1997). | |||||
| REF 33 | Melatonin as modulator of pancreatic enzyme secretion and pancreatoprotector. J Physiol Pharmacol. 2007 Dec;58 Suppl 6:65-80. | |||||
| REF 34 | The ghrelin pentapeptide inhibits the secretion of pancreatic juice in rats. J Physiol Pharmacol. 2006 Dec;57(4):691-700. | |||||
| REF 35 | Effects of intraduodenal administration of tarazepide on pancreatic secretion and duodenal EMG in neonatal calves. Regul Pept. 1998 Nov 30;78(1-3):113-23. | |||||
| REF 36 | SR146131: a new potent, orally active, and selective nonpeptide cholecystokinin subtype 1 receptor agonist. I. In vitro studies. J Pharmacol Exp Ther. 1999 May;289(2):742-51. | |||||
| REF 37 | US patent application no. 8,748,419, Antagonists. | |||||
| REF 38 | Effect of T-0632, a cholecystokininA receptor antagonist, on experimental acute pancreatitis. Jpn J Pharmacol. 1997 Feb;73(2):105-12. | |||||
| REF 39 | Cholecystokinin antagonists: (R)-tryptophan-based hybrid antagonists of high affinity and selectivity for CCK-A receptors. J Med Chem. 1991 Dec;34(12):3350-9. | |||||
| REF 40 | Characterization of two novel cholecystokinin tetrapeptide (30-33) analogues, A-71623 and A-70874, that exhibit high potency and selectivity for cholecystokinin-A receptors. Mol Pharmacol. 1991 Mar;39(3):346-51. | |||||
| REF 41 | Biological properties of (R)-4-benzamido-5-oxopentanoic basic derivatives as CCK-antagonists, Bioorg. Med. Chem. Lett. 3(5):861-866 (1993). | |||||
| REF 42 | Dual CCK-A and CCK-B receptor antagonists (II). Preparation and structure activity relationships of 5-alkyl-9-methyl-1,4-benzodiazepines and discovery of FR208419. Chem Pharm Bull (Tokyo). 2000 Jan;48(1):1-15. | |||||
| REF 43 | Synthesis and stereochemical structure-activity relationships of 1,3-dioxoperhydropyrido[1,2-c]pyrimidine derivatives: potent and selective cholecy... J Med Chem. 1997 Oct 10;40(21):3402-7. | |||||
| REF 44 | Behavioral and neurochemical study on the role of the brain cholecystokinin system in anxiety. Hokkaido Igaku Zasshi. 1998 Sep;73(5):463-73. | |||||
| REF 45 | Selective ligands for cholecystokinin receptor subtypes CCK-A and CCK-B within a single structural class, Bioorg. Med. Chem. Lett. 3(5):881-884 (1993). | |||||
| REF 46 | Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. J Med Chem. 2006 May 18;49(10):2868-75. | |||||
| REF 47 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 76). | |||||
| REF 48 | Hybrid cholecystokinin-A antagonists based on molecular modeling of lorglumide and L-364,718. J Med Chem. 1992 Mar 20;35(6):1042-9. | |||||
| REF 49 | Unexpected relationship between plasma protein binding and the pharmacodynamics of 2-NAP, a CCK1-receptor antagonist. Br J Clin Pharmacol. 2007 May;63(5):618-22. | |||||
| REF 50 | Toward developing peptidomimetics: Successful replacement of backbone amide bonds in tetrapeptide-based CCK-A receptor agonists, Bioorg. Med. Chem. Lett. 3(5):855-860 (1993). | |||||
| REF 51 | Privileged structures: a useful concept for the rational design of new lead drug candidates. Mini Rev Med Chem. 2007 Nov;7(11):1108-19. | |||||
| REF 52 | Role of CCK(A) receptors in postprandial lower esophageal sphincter function in morbidly obese subjects. Dig Dis Sci. 2002 Nov;47(11):2531-7. | |||||
| REF 53 | Synthesis and biological activities of pseudopeptide analogues of the C-terminal heptapeptide of cholecystokinin. On the importance of the peptide ... J Med Chem. 1987 Aug;30(8):1366-73. | |||||
| REF 54 | Synthesis and binding affinities of cyclic and related linear analogues of CCK8 selective for central receptors. J Med Chem. 1989 Jun;32(6):1184-90. | |||||
| REF 55 | Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917-21. | |||||
| REF 56 | Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6728-32. | |||||
| REF 57 | Second generation "peptoid" CCK-B receptor antagonists: identification and development of N-(adamantyloxycarbonyl)-alpha-methyl-(R)-tryptophan derivative (CI-1015) with an improved pharmacokinetic profile. J Med Chem. 1998 Jan 1;41(1):38-45. | |||||
| REF 58 | Discovery of 1,5-benzodiazepines with peripheral cholecystokinin (CCK-A) receptor agonist activity. 1. Optimization of the agonist "trigger". J Med Chem. 1996 Jan 19;39(2):562-9. | |||||
| REF 59 | Optimization of 3-(1H-indazol-3-ylmethyl)-1,5-benzodiazepines as potent, orally active CCK-A agonists. J Med Chem. 1997 Aug 15;40(17):2706-25. | |||||
| REF 60 | Partial retro-inverso, retro, and inverso modifications of hydrazide linked bifunctional peptides for opioid and cholecystokinin (CCK) receptors. J Med Chem. 2007 Jan 11;50(1):165-8. | |||||
| REF 61 | Design and synthesis of novel hydrazide-linked bifunctional peptides as delta/mu opioid receptor agonists and CCK-1/CCK-2 receptor antagonists. J Med Chem. 2006 Mar 9;49(5):1773-80. | |||||
| REF 62 | Pharmacological study of IQM-97,423, a potent and selective CCK1 receptor antagonist with protective effect in experimental acute pancreatitis. Pharmacology. 2004 Oct;72(2):68-76. | |||||
| REF 63 | Identification of a region of the N-terminal of the human CCKA receptor essential for the high affinity interaction with agonist CCK. Biochem Biophys Res Commun. 1995 Aug 24;213(3):845-52. | |||||
| REF 64 | 3-[5-(3,4-Dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionate (JNJ-17156516), a novel, potent, and selective cholecystokini... J Pharmacol Exp Ther. 2007 Nov;323(2):562-9. | |||||
| REF 65 | Effects of KSG-504, a new CCK-A receptor antagonist, on gallbladder and gastrointestinal functions. Nihon Yakurigaku Zasshi. 1996 Jan;107(1):33-44. | |||||
| REF 66 | High-affinity and potent, water-soluble 5-amino-1,4-benzodiazepine CCKB/gastrin receptor antagonists containing a cationic solubilizing group. J Med Chem. 1994 Mar 18;37(6):719-21. | |||||
| REF 67 | Controlled modification of acidity in cholecystokinin B receptor antagonists: N-(1,4-benzodiazepin-3-yl)-N'-[3-(tetrazol-5-ylamino) phenyl]ureas. J Med Chem. 1996 Feb 16;39(4):842-9. | |||||
| REF 68 | C5-piperazinyl-1,4-benzodiazepines, water-soluble, orally bioa vailable CCKB/gastrin receptor antagonists, Bioorg. Med. Chem. Lett. 5(24):3023-3026 (1995). | |||||
| REF 69 | Synthesis and binding affinities of analogues of cholecystokinin-(30-33) as probes for central nervous system cholecystokinin receptors. J Med Chem. 1987 Apr;30(4):729-32. | |||||
| REF 70 | Pharmacological profile of TP-680, a new cholecystokininA receptor antagonist. Br J Pharmacol. 1996 Apr;117(7):1558-64. | |||||
| REF 71 | 2D-QSAR and 3D-QSAR/CoMFA analyses of the N-terminal substituted anthranilic acid based CCK(1) receptor antagonists: 'Hic Rhodus, hic saltus'. Bioorg Med Chem. 2009 Jul 15;17(14):5198-206. | |||||
| REF 72 | Synthesis and evaluation of novel benzimidazole derivative [Bz-Im] and its radio/biological studies. Bioorg Med Chem Lett. 2007 May 15;17(10):2749-55. | |||||
| REF 73 | Anthranilic acid based CCK1 receptor antagonists and CCK-8 have a common step in their "receptor desmodynamic processes". J Med Chem. 2006 Apr 20;49(8):2456-62. | |||||
| REF 74 | Anthranilic acid based CCK1 receptor antagonists: blocking the receptor with the same 'words' of the endogenous ligand. Bioorg Med Chem. 2009 Mar 15;17(6):2336-50. | |||||
| REF 75 | Characterization of the binding of [3H]-(+/-)-L-364,718: a new potent, nonpeptide cholecystokinin antagonist radioligand selective for peripheral receptors. Mol Pharmacol. 1986 Sep;30(3):212-7. | |||||
| REF 76 | Structural insights into human brain-gut peptide cholecystokinin receptors. Cell Discov. 2022 Jun 7;8(1):55. | |||||
| REF 77 | Structures of the human cholecystokinin receptors bound to agonists and antagonists. Nat Chem Biol. 2021 Dec;17(12):1230-1237. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

