Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T40474
(Former ID: TTDC00110)
|
|||||
| Target Name |
Proto-oncogene c-Met (MET)
|
|||||
| Synonyms |
Tyrosine-protein kinase Met; Scatter factor receptor; SF receptor; Met proto-oncogene tyrosine kinase; Hepatocyte growth factor receptor; HGF/SF receptor; HGF-SF receptor; HGF receptor; C-met; C-Met receptor tyrosine kinase
Click to Show/Hide
|
|||||
| Gene Name |
MET
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Lung cancer [ICD-11: 2C25] | |||||
| 2 | Non-small-cell lung cancer [ICD-11: 2C25] | |||||
| 3 | Thyroid cancer [ICD-11: 2D10] | |||||
| Function |
Regulates many physiological processes including proliferation, scattering, morphogenesis and survival. Ligand binding at the cell surface induces autophosphorylation of MET on its intracellular domain that provides docking sites for downstream signaling molecules. Following activation by ligand, interacts with the PI3-kinase subunit PIK3R1, PLCG1, SRC, GRB2, STAT3 or the adapter GAB1. Recruitment of these downstream effectors by MET leads to the activation of several signaling cascades including the RAS-ERK, PI3 kinase-AKT, or PLCgamma-PKC. The RAS-ERK activation is associated with the morphogenetic effects while PI3K/AKT coordinates prosurvival effects. During embryonic development, MET signaling plays a role in gastrulation, development and migration of muscles and neuronal precursors, angiogenesis and kidney formation. In adults, participates in wound healing as well as organ regeneration and tissue remodeling. Promotes also differentiation and proliferation of hematopoietic cells. May regulate cortical bone osteogenesis. Receptor tyrosine kinase that transduces signals from the extracellular matrix into the cytoplasm by binding to hepatocyte growth factor/HGF ligand.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MKAPAVLAPGILVLLFTLVQRSNGECKEALAKSEMNVNMKYQLPNFTAETPIQNVILHEH
HIFLGATNYIYVLNEEDLQKVAEYKTGPVLEHPDCFPCQDCSSKANLSGGVWKDNINMAL VVDTYYDDQLISCGSVNRGTCQRHVFPHNHTADIQSEVHCIFSPQIEEPSQCPDCVVSAL GAKVLSSVKDRFINFFVGNTINSSYFPDHPLHSISVRRLKETKDGFMFLTDQSYIDVLPE FRDSYPIKYVHAFESNNFIYFLTVQRETLDAQTFHTRIIRFCSINSGLHSYMEMPLECIL TEKRKKRSTKKEVFNILQAAYVSKPGAQLARQIGASLNDDILFGVFAQSKPDSAEPMDRS AMCAFPIKYVNDFFNKIVNKNNVRCLQHFYGPNHEHCFNRTLLRNSSGCEARRDEYRTEF TTALQRVDLFMGQFSEVLLTSISTFIKGDLTIANLGTSEGRFMQVVVSRSGPSTPHVNFL LDSHPVSPEVIVEHTLNQNGYTLVITGKKITKIPLNGLGCRHFQSCSQCLSAPPFVQCGW CHDKCVRSEECLSGTWTQQICLPAIYKVFPNSAPLEGGTRLTICGWDFGFRRNNKFDLKK TRVLLGNESCTLTLSESTMNTLKCTVGPAMNKHFNMSIIISNGHGTTQYSTFSYVDPVIT SISPKYGPMAGGTLLTLTGNYLNSGNSRHISIGGKTCTLKSVSNSILECYTPAQTISTEF AVKLKIDLANRETSIFSYREDPIVYEIHPTKSFISGGSTITGVGKNLNSVSVPRMVINVH EAGRNFTVACQHRSNSEIICCTTPSLQQLNLQLPLKTKAFFMLDGILSKYFDLIYVHNPV FKPFEKPVMISMGNENVLEIKGNDIDPEAVKGEVLKVGNKSCENIHLHSEAVLCTVPNDL LKLNSELNIEWKQAISSTVLGKVIVQPDQNFTGLIAGVVSISTALLLLLGFFLWLKKRKQ IKDLGSELVRYDARVHTPHLDRLVSARSVSPTTEMVSNESVDYRATFPEDQFPNSSQNGS CRQVQYPLTDMSPILTSGDSDISSPLLQNTVHIDLSALNPELVQAVQHVVIGPSSLIVHF NEVIGRGHFGCVYHGTLLDNDGKKIHCAVKSLNRITDIGEVSQFLTEGIIMKDFSHPNVL SLLGICLRSEGSPLVVLPYMKHGDLRNFIRNETHNPTVKDLIGFGLQVAKGMKYLASKKF VHRDLAARNCMLDEKFTVKVADFGLARDMYDKEYYSVHNKTGAKLPVKWMALESLQTQKF TTKSDVWSFGVLLWELMTRGAPPYPDVNTFDITVYLLQGRRLLQPEYCPDPLYEVMLKCW HPKAEMRPSFSELVSRISAIFSTFIGEHYVHVNATYVNVKCVAPYPSLLSSEDNADDEVD TRPASFWETS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T92AMR | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 5 Approved Drugs | + | ||||
| 1 | Amivantamab | Drug Info | Approved | Non-small-cell lung cancer | [2] | |
| 2 | Cabozantinib | Drug Info | Approved | Thyroid cancer | [3], [4] | |
| 3 | Capmatinib | Drug Info | Approved | Non-small-cell lung cancer | [5] | |
| 4 | Crizotinib | Drug Info | Approved | Non-small-cell lung cancer | [6], [7] | |
| 5 | Tepotinib | Drug Info | Approved | Non-small-cell lung cancer | [8] | |
| Clinical Trial Drug(s) | [+] 41 Clinical Trial Drugs | + | ||||
| 1 | Beperminogene perplasmid | Drug Info | Phase 3 | Heart disease | [9] | |
| 2 | RG3638 | Drug Info | Phase 3 | Non-small-cell lung cancer | [10] | |
| 3 | Savolitinib | Drug Info | Phase 3 | Renal cell carcinoma | [11] | |
| 4 | Tivantinib | Drug Info | Phase 3 | Solid tumour/cancer | [12], [13] | |
| 5 | MGCD516 | Drug Info | Phase 2/3 | Solid tumour/cancer | [14] | |
| 6 | AMG 208 | Drug Info | Phase 2 | Solid tumour/cancer | [15] | |
| 7 | AMG 337 | Drug Info | Phase 2 | Solid tumour/cancer | [16] | |
| 8 | APL-101 | Drug Info | Phase 2 | Non-small-cell lung cancer | [17] | |
| 9 | CMX-2043 | Drug Info | Phase 2 | Myocardial reperfusion injury | [18] | |
| 10 | Emibetuzumab | Drug Info | Phase 2 | Solid tumour/cancer | [19] | |
| 11 | HM-5016504 | Drug Info | Phase 2 | Solid tumour/cancer | [20] | |
| 12 | LY-2875358 | Drug Info | Phase 2 | Solid tumour/cancer | [21] | |
| 13 | LY2801653 | Drug Info | Phase 2 | Solid tumour/cancer | [22] | |
| 14 | SAR-125844 | Drug Info | Phase 2 | Solid tumour/cancer | [23] | |
| 15 | Telisotuzumab vedotin | Drug Info | Phase 2 | Non-small-cell lung cancer | [24] | |
| 16 | XL880 | Drug Info | Phase 2 | Squamous head and neck cell carcinom | [25], [26] | |
| 17 | Anti-C-met CAR-T cells | Drug Info | Phase 1/2 | Colorectal cancer | [27] | |
| 18 | ChronSeal | Drug Info | Phase 1/2 | Fibrosis | [28] | |
| 19 | MK-2461 | Drug Info | Phase 1/2 | Alzheimer disease | [29] | |
| 20 | Sym015 | Drug Info | Phase 1/2 | Solid tumour/cancer | [11], [19] | |
| 21 | ABBV-399 | Drug Info | Phase 1 | Solid tumour/cancer | [30] | |
| 22 | ABT-700 | Drug Info | Phase 1 | Advanced solid tumour | [31] | |
| 23 | Altiratinib | Drug Info | Phase 1 | Solid tumour/cancer | [32] | |
| 24 | Autologous T Cells Expressing MET scFv CAR | Drug Info | Phase 1 | Melanoma | [33] | |
| 25 | AZD9592 | Drug Info | Phase 1 | Aggressive cancer | [34] | |
| 26 | BPI-9016 M | Drug Info | Phase 1 | Solid tumour/cancer | [35] | |
| 27 | C-Met/PD-L1 CAR-T Cell | Drug Info | Phase 1 | Hepatocellular carcinoma | [36] | |
| 28 | CBT-101 | Drug Info | Phase 1 | Solid tumour/cancer | [11] | |
| 29 | E-7050 | Drug Info | Phase 1 | Head and neck cancer | [37], [38] | |
| 30 | EMD-1204831 | Drug Info | Phase 1 | Solid tumour/cancer | [39] | |
| 31 | Hepapoietin | Drug Info | Phase 1 | Liver disease | [40] | |
| 32 | JNJ-38877605 | Drug Info | Phase 1 | Solid tumour/cancer | [41] | |
| 33 | LY3164530 | Drug Info | Phase 1 | Advanced cancer | [42] | |
| 34 | MK-8033 | Drug Info | Phase 1 | Solid tumour/cancer | [43] | |
| 35 | PF-4217903 | Drug Info | Phase 1 | Solid tumour/cancer | [44] | |
| 36 | REGN5093 | Drug Info | Phase 1 | Non-small-cell lung cancer | [45] | |
| 37 | RXDX-106 | Drug Info | Phase 1 | Solid tumour/cancer | [46] | |
| 38 | SGX523 | Drug Info | Phase 1 | Solid tumour/cancer | [47], [48] | |
| 39 | TPX-0022 | Drug Info | Phase 1 | Solid tumour/cancer | [49] | |
| 40 | TR1801-ADC | Drug Info | Phase 1 | Solid tumour/cancer | [50] | |
| 41 | XL092 | Drug Info | Phase 1 | Solid tumour/cancer | [51] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | Mteron-F1 | Drug Info | Preclinical | Mucositis | [52] | |
| 2 | NPS-1034 | Drug Info | Preclinical | Solid tumour/cancer | [53] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Inhibitor | [+] 49 Inhibitor drugs | + | ||||
| 1 | Amivantamab | Drug Info | [54] | |||
| 2 | Cabozantinib | Drug Info | [4], [55] | |||
| 3 | Savolitinib | Drug Info | [11] | |||
| 4 | Tivantinib | Drug Info | [12] | |||
| 5 | MGCD516 | Drug Info | [57] | |||
| 6 | MGCD516 | Drug Info | [58] | |||
| 7 | APL-101 | Drug Info | [61] | |||
| 8 | Emibetuzumab | Drug Info | [19] | |||
| 9 | HM-5016504 | Drug Info | [57] | |||
| 10 | SAR-125844 | Drug Info | [67] | |||
| 11 | BMS-777607 | Drug Info | [70] | |||
| 12 | ChronSeal | Drug Info | [71] | |||
| 13 | MK-2461 | Drug Info | [29] | |||
| 14 | Sym015 | Drug Info | [11] | |||
| 15 | ABBV-399 | Drug Info | [19] | |||
| 16 | Altiratinib | Drug Info | [55], [73] | |||
| 17 | BPI-9016 M | Drug Info | [75] | |||
| 18 | CBT-101 | Drug Info | [11] | |||
| 19 | EMD-1204831 | Drug Info | [77] | |||
| 20 | Hepapoietin | Drug Info | [78] | |||
| 21 | JNJ-38877605 | Drug Info | [79] | |||
| 22 | MK-8033 | Drug Info | [81] | |||
| 23 | PF-4217903 | Drug Info | [82] | |||
| 24 | REGN5093 | Drug Info | [83] | |||
| 25 | RXDX-106 | Drug Info | [84] | |||
| 26 | SGX523 | Drug Info | [85] | |||
| 27 | TPX-0022 | Drug Info | [86] | |||
| 28 | XL092 | Drug Info | [88] | |||
| 29 | NPS-1034 | Drug Info | [89] | |||
| 30 | 1-(2-nitrophenethyl)-1H-pyrrolo[3,2-b]pyridine | Drug Info | [90] | |||
| 31 | 1-(phenylsulfonyl)-1H-pyrrolo[3,2-b]pyridine | Drug Info | [90] | |||
| 32 | 1-benzyl-1H-pyrrolo[3,2-b]pyridine | Drug Info | [90] | |||
| 33 | 3-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine | Drug Info | [90] | |||
| 34 | AM7 | Drug Info | [91] | |||
| 35 | Anti-cMET mab | Drug Info | [57] | |||
| 36 | APS-3010 | Drug Info | [57] | |||
| 37 | BAY-85-3474 | Drug Info | [57] | |||
| 38 | BMS-536924 | Drug Info | [92] | |||
| 39 | CMET Avimer polypeptides | Drug Info | [57] | |||
| 40 | DP-3590 | Drug Info | [57] | |||
| 41 | PF-00614435 | Drug Info | [57] | |||
| 42 | PHA-665752 | Drug Info | [57] | |||
| 43 | PMID21123062C27 | Drug Info | [94] | |||
| 44 | PMID21967808CR-16 | Drug Info | [95] | |||
| 45 | PMID24210504C1o | Drug Info | [96] | |||
| 46 | PRS-110 | Drug Info | [57] | |||
| 47 | RP-1040 | Drug Info | [57] | |||
| 48 | SU11274 | Drug Info | [97] | |||
| 49 | TP-801 | Drug Info | [57] | |||
| Modulator | [+] 14 Modulator drugs | + | ||||
| 1 | Capmatinib | Drug Info | [5] | |||
| 2 | Crizotinib | Drug Info | [1] | |||
| 3 | Tepotinib | Drug Info | [8] | |||
| 4 | Beperminogene perplasmid | Drug Info | [56] | |||
| 5 | RG3638 | Drug Info | [57] | |||
| 6 | AMG 208 | Drug Info | [59] | |||
| 7 | AMG 337 | Drug Info | [60] | |||
| 8 | CMX-2043 | Drug Info | [62] | |||
| 9 | LY2801653 | Drug Info | [65], [66] | |||
| 10 | XL880 | Drug Info | [69] | |||
| 11 | ABT-700 | Drug Info | [72] | |||
| 12 | E-7050 | Drug Info | [76] | |||
| 13 | LY3164530 | Drug Info | [80] | |||
| 14 | GE-137 | Drug Info | [93] | |||
| CAR-T-Cell-Therapy | [+] 2 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Anti-C-met CAR-T cells | Drug Info | [27] | |||
| 2 | Autologous T Cells Expressing MET scFv CAR | Drug Info | [33] | |||
| CAR-T-Cell-Therapy(Dual specific) | [+] 1 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | C-Met/PD-L1 CAR-T Cell | Drug Info | [36] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | Mteron-F1 | Drug Info | [52] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Adenosine triphosphate | Ligand Info | |||||
| Structure Description | Structure of MET receptor tyrosine kinase in complex with ATP | PDB:3DKC | ||||
| Method | X-ray diffraction | Resolution | 1.52 Å | Mutation | Yes | [99] |
| PDB Sequence |
VHIDLSALNP
1060 ELVQAVQHVV1070 IGPSSLIVHF1080 NEVIGRGHFG1090 CVYHGTLLDN1100 DGKKIHCAVK 1110 SLNRITDIGE1120 VSQFLTEGII1130 MKDFSHPNVL1140 SLLGICLRSE1150 GSPLVVLPYM 1160 KHGDLRNFIR1170 NETHNPTVKD1180 LIGFGLQVAK1190 GMKFLASKKF1200 VHRDLAARNC 1210 MLDEKFTVKV1220 ADFGLARDMY1230 DKEFDSVHNK1240 TGAKLPVKWM1250 ALESLQTQKF 1260 TTKSDVWSFG1270 VLLWELMTRG1280 APPYPDVNTF1290 DITVYLLQGR1300 RLLQPEYCPD 1310 PLYEVMLKCW1320 HPKAEMRPSF1330 SELVSRISAI1340 FSTFIGEHYV1350 HVNATYVNVK 1360 EG
|
|||||
|
|
ILE1084
3.377
GLY1085
3.534
ARG1086
3.912
GLY1087
3.138
HIS1088
2.665
PHE1089
3.872
GLY1090
3.501
CYS1091
4.829
VAL1092
3.428
ALA1108
3.480
LYS1110
2.835
|
|||||
| Ligand Name: Crizotinib | Ligand Info | |||||
| Structure Description | X-ray Structure of PF-02341066 bound to the kinase domain of c-Met | PDB:2WGJ | ||||
| Method | X-ray diffraction | Resolution | 2.00 Å | Mutation | No | [100] |
| PDB Sequence |
HIDLSALNPE
1061 LVQAVQHVVI1071 GPSSLIVHFN1081 EVIGRGHFGC1091 VYHGTLLKKI1105 HCAVKSLNRI 1115 TDIGEVSQFL1125 TEGIIMKDFS1135 HPNVLSLLGI1145 CLRSEGSPLV1155 VLPYMKHGDL 1165 RNFIRNETHN1175 PTVKDLIGFG1185 LQVAKGMKYL1195 ASKKFVHRDL1205 AARNCMLDEK 1215 FTVKVADFGL1225 ARDMYDKEYY1235 SVHNKTGAKL1245 PVKWMALESL1255 QTQKFTTKSD 1265 VWSFGVLLWE1275 LMTRGAPPYP1285 DVNTFDITVY1295 LLQGRRLLQP1305 EYCPDPLYEV 1315 MLKCWHPKAE1325 MRPSFSELVS1335 RISAIFSTFI1345
|
|||||
|
|
ILE1084
3.465
GLY1085
3.681
VAL1092
3.762
ALA1108
3.265
LYS1110
4.690
LEU1140
3.848
LEU1157
3.692
PRO1158
3.051
TYR1159
3.435
MET1160
2.910
LYS1161
3.865
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
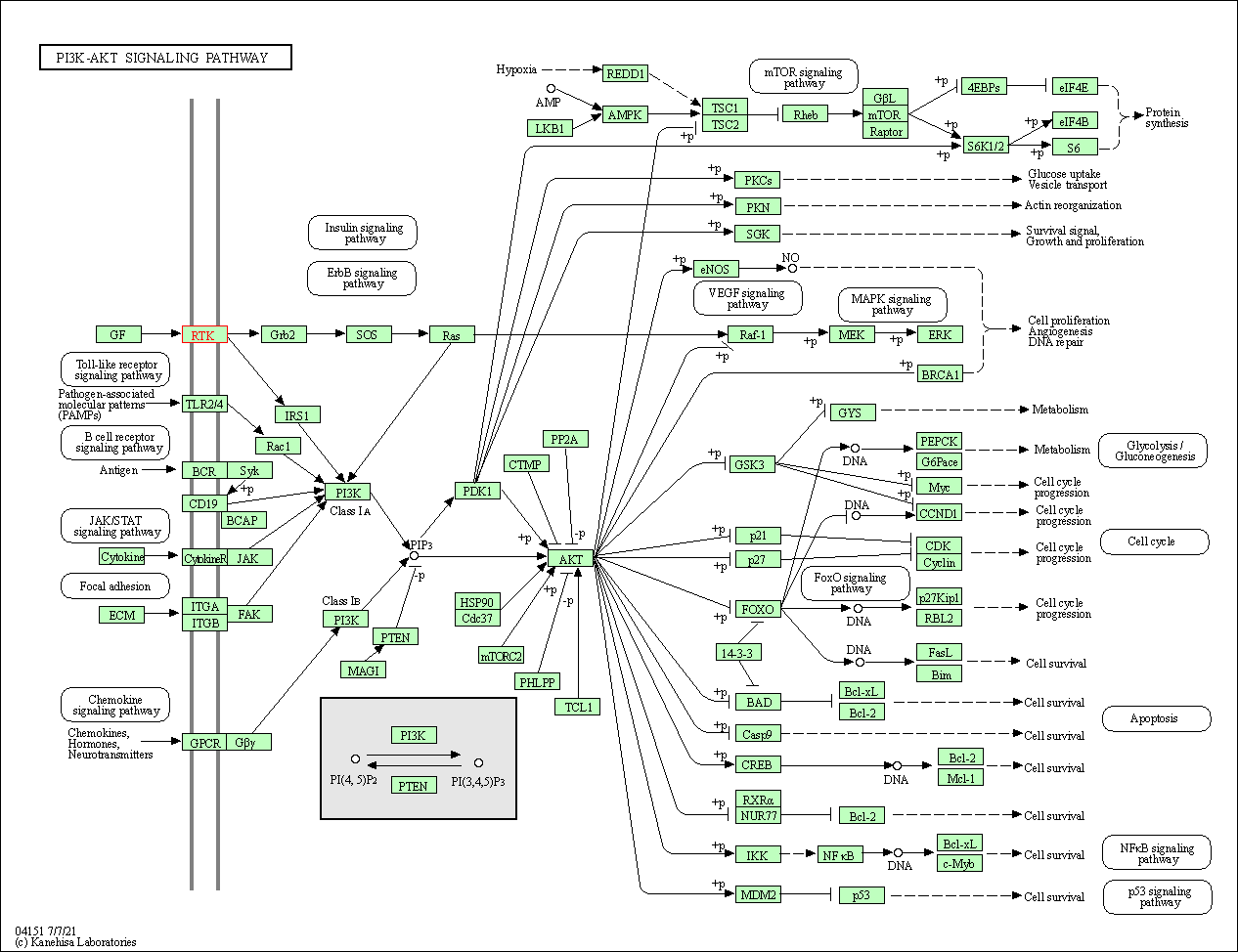
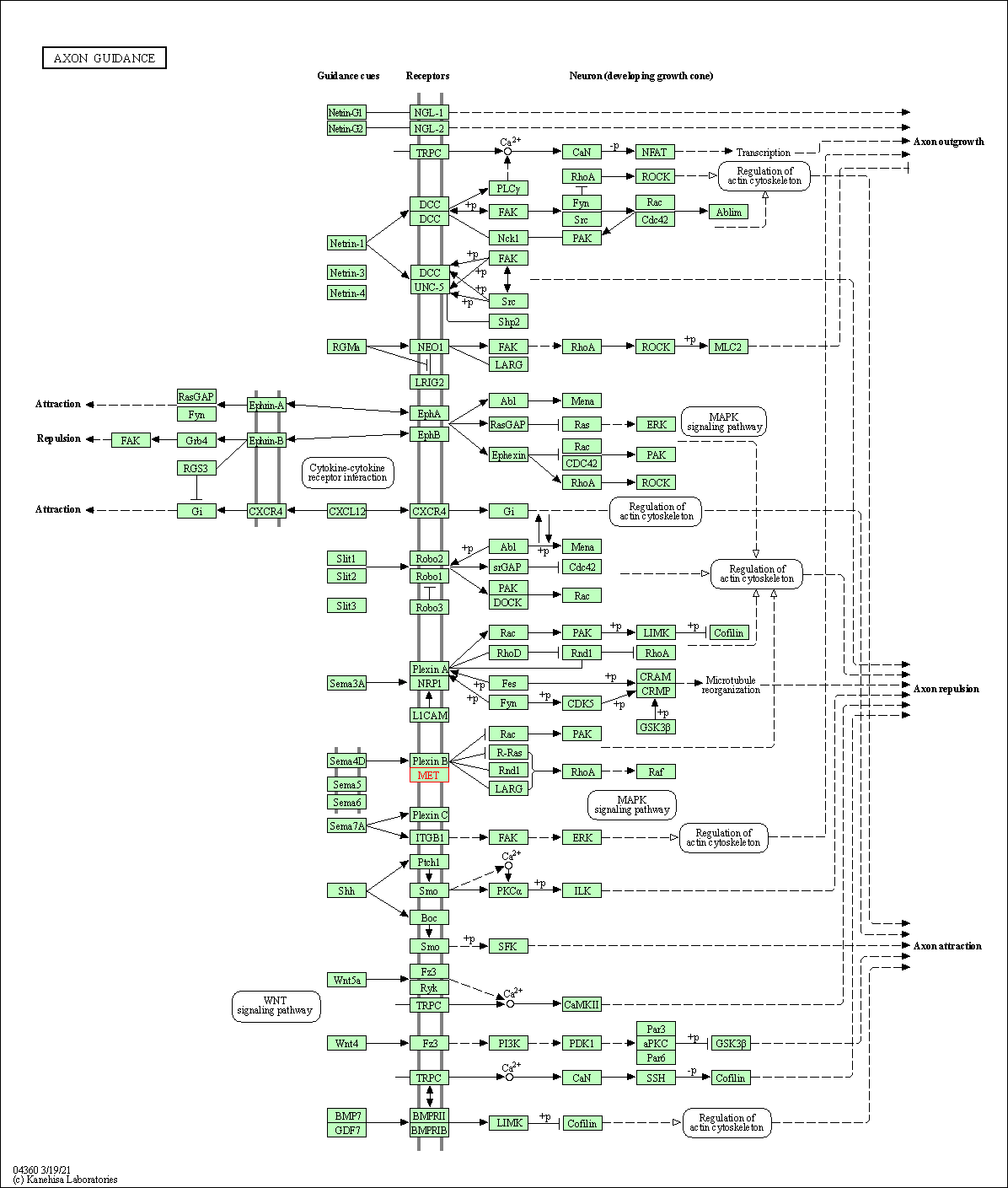
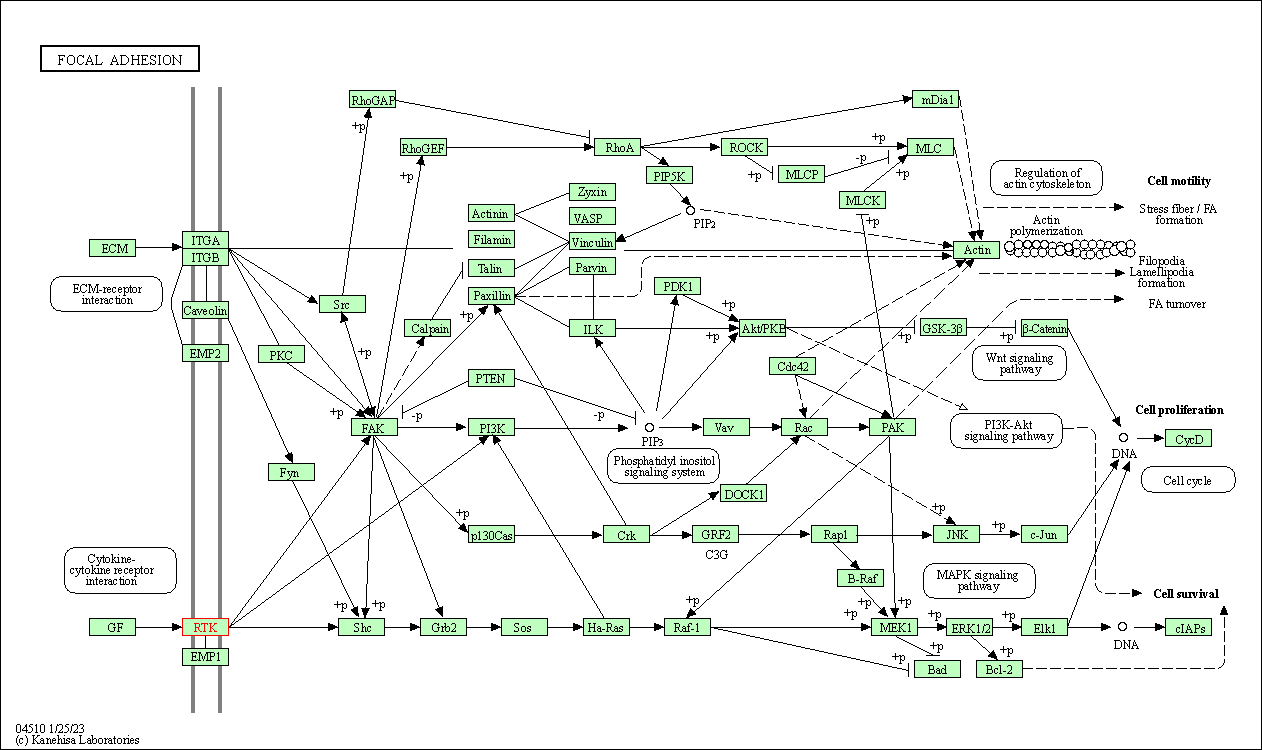
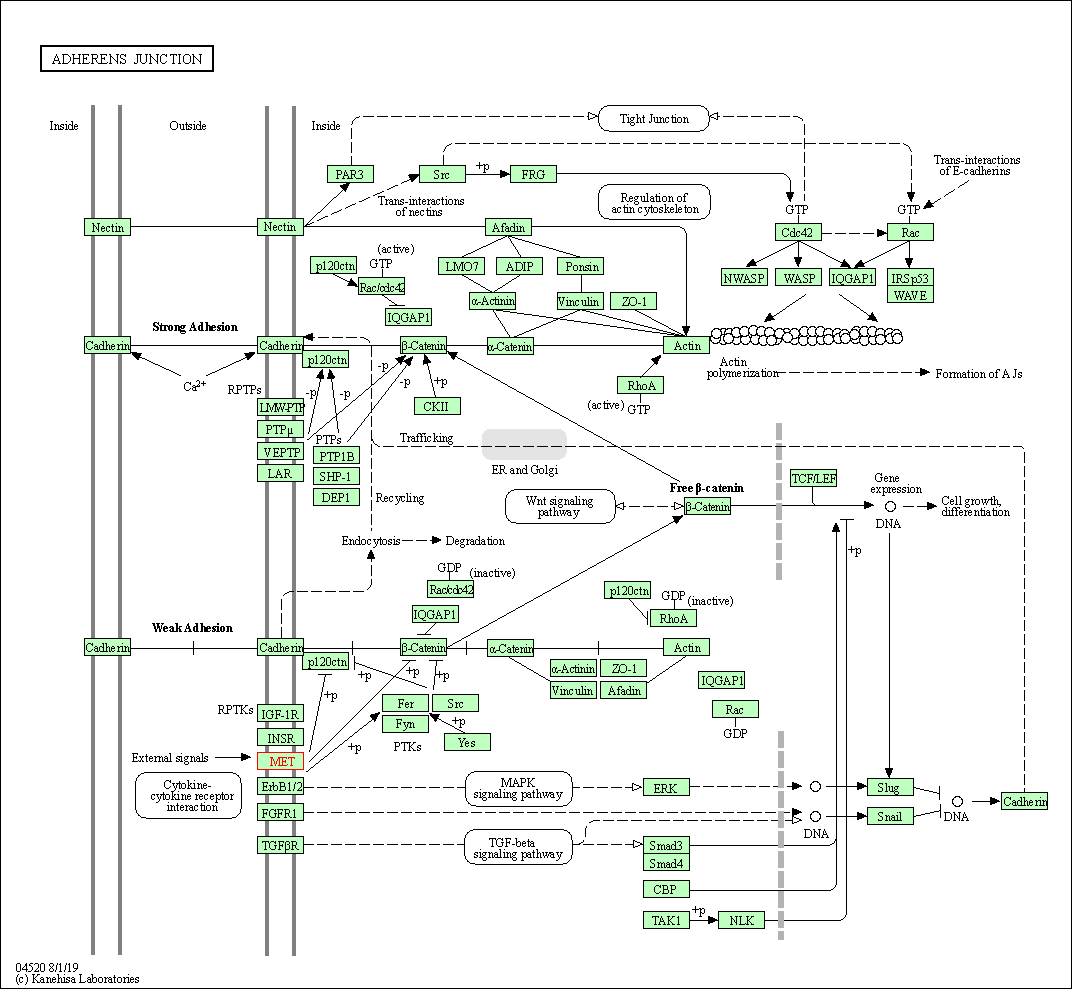
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Rap1 signaling pathway | hsa04015 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Axon guidance | hsa04360 | Affiliated Target |

|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| Focal adhesion | hsa04510 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Adherens junction | hsa04520 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 35 | Degree centrality | 3.76E-03 | Betweenness centrality | 2.68E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.51E-01 | Radiality | 1.44E+01 | Clustering coefficient | 2.22E-01 |
| Neighborhood connectivity | 5.52E+01 | Topological coefficient | 6.69E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 761210. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5887). | |||||
| REF 4 | Clinical pipeline report, company report or official report of Exelixis (2011). | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4903). | |||||
| REF 7 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2021 | |||||
| REF 9 | ClinicalTrials.gov (NCT02144610) Safety and Efficacy of Subjects With Critical Limb Ischemia. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT02488330) An Extension Study of Onartuzumab in Patients With Solid Tumors on Study Treatment Previously Enrolled in a Company Sponsored Study. | |||||
| REF 11 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7948). | |||||
| REF 13 | A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014 Apr;32(2):355-61. | |||||
| REF 14 | ClinicalTrials.gov (NCT04887870) Study of Sitravatinib With or Without Other Anticancer Therapies Receiving Clinical Benefit From Parent Study. U.S. National Institutes of Health. | |||||
| REF 15 | ClinicalTrials.gov (NCT02420587) AMG 208 Tumor Microenvironment in Metastatic Castration Resistant Prostate Cancer (mCRPC). U.S. National Institutes of Health. | |||||
| REF 16 | ClinicalTrials.gov (NCT02016534) Phase 2 Study of AMG 337 in MET Amplified Gastric/Esophageal Adenocarcinoma or Other Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT04258033) A Study of PLB1001 in Non-small Cell Lung Cancer With c-Met Dysregulation. U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT02103959) Safety and Efficacy of CMX-2043 for Protection of the Heart and Kidneys in Subjects Undergoing Coronary Angiography. U.S. National Institutes of Health. | |||||
| REF 19 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 20 | ClinicalTrials.gov (NCT02127710) A Phase II Trial to Evaluate the Efficacy of AZD6094 (HMPL-504) in Patients With Papillary Renal Cell Carcinoma (PRCC). U.S. National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT01900652) A Study of LY2875358 in Non Small Cell Lung Cancer (NSCLC) Participants. U.S. National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT01285037) A Study of LY2801653 in Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 23 | ClinicalTrials.gov (NCT02435121) A Study Assessing Efficacy and Safety of SAR125844 in NSCLC Patients With MET Amplification. U.S. National Institutes of Health. | |||||
| REF 24 | ClinicalTrials.gov (NCT03539536) Study of Telisotuzumab Vedotin (ABBV-399) in Participants With Previously Treated c-Met+ Non-Small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5679). | |||||
| REF 26 | Clinical pipeline report, company report or official report of Exelixis (2011). | |||||
| REF 27 | ClinicalTrials.gov (NCT03638206) Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | |||||
| REF 28 | ClinicalTrials.gov (NCT00797706) Phase I/II Dose Ranging CHRONSEAL Study in Venous Leg Ulcers. U.S. National Institutes of Health. | |||||
| REF 29 | MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 2010 Feb 15;70(4):1524-33. | |||||
| REF 30 | ClinicalTrials.gov (NCT02099058) A Phase 1/1b Study With ABBV-399, an Antibody Drug Conjugate, in Subjects With Advanced Solid Cancer Tumors. U.S. National Institutes of Health. | |||||
| REF 31 | ClinicalTrials.gov (NCT01472016) Study of ABT-700 in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800040094) | |||||
| REF 33 | ClinicalTrials.gov (NCT03060356) Autologous T Cells Expressing MET scFv CAR (RNA CART-cMET) | |||||
| REF 34 | ClinicalTrials.gov (NCT05647122) A Phase I, Multicenter, Open-label, First-in-Human, Dose Escalation and Expansion Study of AZD9592 as Monotherapy and in Combination With Anti-cancer Agents in Patients With Advanced Solid Tumors. U.S.National Institutes of Health. | |||||
| REF 35 | ClinicalTrials.gov (NCT02478866) Safety and Pharmacokinetics of BPI-9016M in Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 36 | ClinicalTrials.gov (NCT03672305) Clinical Study on the Efficacy and Safety of c-Met/PD-L1 CAR-T Cell Injection in the Treatment of HCC | |||||
| REF 37 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7956). | |||||
| REF 38 | ClinicalTrials.gov (NCT02533102) Pharmacokinetics and Food Effect of Single Oral Dose of E7050 in Healthy Volunteers. | |||||
| REF 39 | ClinicalTrials.gov (NCT01110083) First-in-Man, Dose-escalation Trial of c-Met Kinase Inhibitor EMD 1204831 in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 40 | Pharmacokinetics and biochemical effects of hepapoietin in patients with chronic liver disease. Aliment Pharmacol Ther. 2002 Feb;16(2):235-42. | |||||
| REF 41 | ClinicalTrials.gov (NCT00651365) A Safety and Dose-finding Study of JNJ-38877605 in Patients With Advanced or Refractory Solid Tumors.. U.S. National Institutes of Health. | |||||
| REF 42 | ClinicalTrials.gov (NCT02221882) A Study of LY3164530 in Participants With Cancer | |||||
| REF 43 | ClinicalTrials.gov (NCT00559182) A Study of MK-8033 in Patients With Advanced Solid Tumors (MK-8033-001). U.S. National Institutes of Health. | |||||
| REF 44 | ClinicalTrials.gov (NCT00706355) A Study of PF-04217903 in Patients With Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 45 | ClinicalTrials.gov (NCT04077099) REGN5093 in Patients With MET-Altered Advanced Non-Small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 46 | ClinicalTrials.gov (NCT03454243) Oral Immunomodulatory Tyrosine Kinase Inhibitor in Patients With Locally Advanced or Metastatic Solid Tumors (TITAN). U.S. National Institutes of Health. | |||||
| REF 47 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5709). | |||||
| REF 48 | ClinicalTrials.gov (NCT00607399) Safety Study of SGX523, a Small Molecule Met Inhibitor, to Treat Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 49 | ClinicalTrials.gov (NCT03993873) Phase 1 Study of TPX-0022, a MET/CSF1R/SRC Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in MET. U.S. National Institutes of Health. | |||||
| REF 50 | ClinicalTrials.gov (NCT03859752) TR1801-ADC in Patients With Tumors That Express c-Met. U.S. National Institutes of Health. | |||||
| REF 51 | ClinicalTrials.gov (NCT03845166) A Study of XL092 as Single-Agent and Combination Therapy in Subjects With Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 52 | Emerging drugs for chemotherapy-induced mucositis. Expert Opin Emerg Drugs. 2008 Sep;13(3):511-22. | |||||
| REF 53 | AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019 Nov 4;18(1):153. | |||||
| REF 54 | Antitumor Activity of Amivantamab (JNJ-61186372), an EGFR-MET Bispecific Antibody, in Diverse Models of EGFR Exon 20 Insertion-Driven NSCLC. Cancer Discov. 2020 Aug;10(8):1194-1209. | |||||
| REF 55 | Tropomyosin receptor kinase inhibitors: an updated patent review for 2010-2016 - Part I.Expert Opin Ther Pat. 2017 Jun;27(6):733-751. | |||||
| REF 56 | Beperminogene perplasmid for the treatment of critical limb ischemia. Expert Rev Cardiovasc Ther. 2014 Oct;12(10):1145-56. | |||||
| REF 57 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1815). | |||||
| REF 58 | Role and relevance of TrkB mutations and expression in non-small cell lung cancer. Clin Cancer Res. 2011 May 1;17(9):2638-45. | |||||
| REF 59 | A first-in-human study of AMG 208, an oral MET inhibitor, in adult patients with advanced solid tumors. Oncotarget. 2015 Jul 30;6(21):18693-706. | |||||
| REF 60 | J Clin Oncol 32:5s, 2014 (suppl; abstr 2508). | |||||
| REF 61 | Clinical pipeline report, company report or official report of Apollomics. | |||||
| REF 62 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 63 | Clinical pipeline report, company report or official report of Eli Lilly. | |||||
| REF 64 | LY2875358, a neutralizing and internalizing anti-MET bivalent antibody, inhibits HGF-dependent and HGF-independent MET activation and tumor growth. Clin Cancer Res. 2014 Dec 1;20(23):6059-70. | |||||
| REF 65 | Clinical pipeline report, company report or official report of Eli Lilly. | |||||
| REF 66 | LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest New Drugs. 2013 Aug;31(4):833-44. | |||||
| REF 67 | The selective intravenous inhibitor of the MET tyrosine kinase SAR125844 inhibits tumor growth in MET-amplified cancer. Mol Cancer Ther. 2015 Feb;14(2):384-94. | |||||
| REF 68 | Clinical pipeline report, company report or official report of AbbVie. | |||||
| REF 69 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 70 | Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607... J Med Chem. 2009 Mar 12;52(5):1251-4. | |||||
| REF 71 | Clinical pipeline report, company report or official report of Kringle Pharma. | |||||
| REF 72 | J Clin Oncol 32:5s, 2014 (suppl; abstr 2507). | |||||
| REF 73 | Clinical pipeline report, company report or official report of Deciphera Pharmaceuticals. | |||||
| REF 74 | Clinical pipeline report, company report or official report of AstraZeneca | |||||
| REF 75 | First-in-human phase I study of BPI-9016M, a dual MET/Axl inhibitor, in patients with non-small cell lung cancer. J Hematol Oncol. 2020 Jan 16;13(1):6. | |||||
| REF 76 | E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci. 2010 Jan;101(1):210-5. | |||||
| REF 77 | 2012 ASCO Annual Meeting. | |||||
| REF 78 | Pharmacokinetics and biochemical effects of hepapoietin in patients with chronic liver disease. Alimentary Pharmacology & Therapeutics Volume 16, Issue 2, pages 235-242, February 2002. | |||||
| REF 79 | The c-Met Tyrosine Kinase Inhibitor JNJ-38877605 Causes Renal Toxicity through Species-Specific Insoluble Metabolite Formation. Clin Cancer Res. 2015 May 15;21(10):2297-304. | |||||
| REF 80 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 81 | A novel c-Met inhibitor, MK8033, synergizes with carboplatin plus paclitaxel to inhibit ovarian cancer cell growth. Oncol Rep. 2013 May;29(5):2011-8. | |||||
| REF 82 | Sensitivity of selected human tumor models to PF-04217903, a novel selective c-Met kinase inhibitor. Mol Cancer Ther. 2012 Apr;11(4):1036-47. | |||||
| REF 83 | Clinical pipeline report, company report or official report of Regeneron Pharmaceuticals. | |||||
| REF 84 | National Cancer Institute Drug Dictionary (drug name RXDX106). | |||||
| REF 85 | CenterWatch. Drugs in Clinical Trials Database. CenterWatch. 2008. | |||||
| REF 86 | Clinical pipeline report, company report or official report of Turning Point Therapeutics. | |||||
| REF 87 | TR1801-ADC: a highly potent cMet antibody-drug conjugate with high activity in patient-derived xenograft models of solid tumors. Mol Oncol. 2020 Jan;14(1):54-68. | |||||
| REF 88 | Clinical pipeline report, company report or official report of Exelixis. | |||||
| REF 89 | NPS-1034, a novel MET inhibitor, inhibits the activated MET receptor and its constitutively active mutants. Invest New Drugs. 2014 Jun;32(3):389-99. | |||||
| REF 90 | Discovery of 4-azaindoles as novel inhibitors of c-Met kinase. Bioorg Med Chem Lett. 2009 May 15;19(10):2780-4. | |||||
| REF 91 | c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J Biol Chem. 2008 Feb 1;283(5):2675-83. | |||||
| REF 92 | Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitum... J Med Chem. 2005 Sep 8;48(18):5639-43. | |||||
| REF 93 | Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med. 2015 Aug;21(8):955-61. | |||||
| REF 94 | Fused bicyclic derivatives of 2,4-diaminopyrimidine as c-Met inhibitors. Bioorg Med Chem Lett. 2011 Jan 1;21(1):164-7. | |||||
| REF 95 | Improvement in oral bioavailability of 2,4-diaminopyrimidine c-Met inhibitors by incorporation of a 3-amidobenzazepin-2-one group. Bioorg Med Chem. 2011 Nov 1;19(21):6274-84. | |||||
| REF 96 | Design and synthesis of novel 3-(benzo[d]oxazol-2-yl)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine derivatives as selective G-protein-coupled receptor kinase-2 and -5 inhibitors. Bioorg Med Chem Lett. 2013 Dec 15;23(24):6711-6. | |||||
| REF 97 | Potent and selective inhibitors of the Met [hepatocyte growth factor/scatter factor (HGF/SF) receptor] tyrosine kinase block HGF/SF-induced tumor cell growth and invasion. Mol Cancer Ther. 2003 Nov;2(11):1085-92. | |||||
| REF 98 | Clinical pipeline report, company report or official report of Biocytogen | |||||
| REF 99 | SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol Cancer Ther. 2009 Dec;8(12):3181-90. | |||||
| REF 100 | Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem. 2011 Sep 22;54(18):6342-63. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

