Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T45758
(Former ID: TTDC00188)
|
|||||
| Target Name |
CD40L receptor (CD40)
|
|||||
| Synonyms |
Tumor necrosis factor receptor superfamily member 5; TNFRSF5; CDw40; Bp50; B-cell surfaceantigen CD40; B-cell surface antigen CD40
Click to Show/Hide
|
|||||
| Gene Name |
CD40
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 15 Target-related Diseases | + | ||||
| 1 | Diabetes mellitus [ICD-11: 5A10] | |||||
| 2 | Lupus erythematosus [ICD-11: 4A40] | |||||
| 3 | Motor neuron disease [ICD-11: 8B60] | |||||
| 4 | Multiple sclerosis [ICD-11: 8A40] | |||||
| 5 | Myasthenia gravis [ICD-11: 8C6Y] | |||||
| 6 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| 7 | Sjogren syndrome [ICD-11: 4A43] | |||||
| 8 | Thyrotoxicosis [ICD-11: 5A02] | |||||
| 9 | Transplant rejection [ICD-11: NE84] | |||||
| 10 | Brain cancer [ICD-11: 2A00] | |||||
| 11 | Crohn disease [ICD-11: DD70] | |||||
| 12 | Melanoma [ICD-11: 2C30] | |||||
| 13 | Ovarian cancer [ICD-11: 2C73] | |||||
| 14 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 15 | Thrombocytopenia [ICD-11: 3B64] | |||||
| Function |
Transduces TRAF6- and MAP3K8-mediated signals that activate ERK in macrophages and B cells, leading to induction of immunoglobulin secretion. Receptor for TNFSF5/CD40LG.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MVRLPLQCVLWGCLLTAVHPEPPTACREKQYLINSQCCSLCQPGQKLVSDCTEFTETECL
PCGESEFLDTWNRETHCHQHKYCDPNLGLRVQQKGTSETDTICTCEEGWHCTSEACESCV LHRSCSPGFGVKQIATGVSDTICEPCPVGFFSNVSSAFEKCHPWTSCETKDLVVQQAGTN KTDVVCGPQDRLRALVVIPIIFGILFAILLVLVFIKKVAKKPTNKAPHPKQEPQEINFPD DLPGSNTAAPVQETLHGCQPVTQEDGKESRISVQERQ Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T11R4S | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 17 Clinical Trial Drugs | + | ||||
| 1 | APX005M | Drug Info | Phase 2 | Melanoma | [2] | |
| 2 | ASKP-1240 | Drug Info | Phase 2 | Transplant rejection | [3] | |
| 3 | AT-1501 | Drug Info | Phase 2 | Amyotrophic lateral sclerosis | [4] | |
| 4 | BI 655064 | Drug Info | Phase 2 | Lupus | [5] | |
| 5 | CFZ533 | Drug Info | Phase 2 | Rheumatoid arthritis | [6] | |
| 6 | Dazodalibep | Drug Info | Phase 2 | Kidney transplant rejection | [7] | |
| 7 | Frexalimab | Drug Info | Phase 2 | Multiple sclerosis | [8] | |
| 8 | Iscalimab | Drug Info | Phase 2 | Type-1 diabetes | [9] | |
| 9 | KPL-404 | Drug Info | Phase 2 | Rheumatoid arthritis | [10] | |
| 10 | ABBV-323 | Drug Info | Phase 1 | Crohn disease | [5] | |
| 11 | CDX-1140 | Drug Info | Phase 1 | Solid tumour/cancer | [11] | |
| 12 | CP-870,893 | Drug Info | Phase 1 | Solid tumour/cancer | [12] | |
| 13 | GEN1042 | Drug Info | Phase 1 | Solid tumour/cancer | [13] | |
| 14 | NG-350A | Drug Info | Phase 1 | Solid tumour/cancer | [14] | |
| 15 | RG6189 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| 16 | SL-172154 | Drug Info | Phase 1 | Ovarian cancer | [16] | |
| 17 | YH003 | Drug Info | Phase 1 | Aggressive cancer | [17] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | HuCD40L | Drug Info | Terminated | Solid tumour/cancer | [18], [19] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Agonist | [+] 7 Agonist drugs | + | ||||
| 1 | APX005M | Drug Info | [11], [20] | |||
| 2 | CDX-1140 | Drug Info | [25] | |||
| 3 | CP-870,893 | Drug Info | [26] | |||
| 4 | GEN1042 | Drug Info | [27] | |||
| 5 | NG-350A | Drug Info | [28] | |||
| 6 | RG6189 | Drug Info | [15] | |||
| 7 | Human CD40 agonist ligand | Drug Info | [1] | |||
| Inhibitor | [+] 7 Inhibitor drugs | + | ||||
| 1 | AT-1501 | Drug Info | [4] | |||
| 2 | BI 655064 | Drug Info | [5] | |||
| 3 | CFZ533 | Drug Info | [5], [21] | |||
| 4 | Dazodalibep | Drug Info | [22] | |||
| 5 | Iscalimab | Drug Info | [23] | |||
| 6 | KPL-404 | Drug Info | [24] | |||
| 7 | SL-172154 | Drug Info | [29] | |||
| Antagonist | [+] 3 Antagonist drugs | + | ||||
| 1 | Frexalimab | Drug Info | [8] | |||
| 2 | ABBV-323 | Drug Info | [5] | |||
| 3 | Anti-CD40-XTEN | Drug Info | [1] | |||
| Binder | [+] 3 Binder drugs | + | ||||
| 1 | HuCD40L | Drug Info | [31] | |||
| 2 | RhuCD40L | Drug Info | [32] | |||
| 3 | CD154 | Drug Info | [33] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Activation-inducible TNFR family receptor (TNFRSF18) | 27.966 (33/118) | 5.00E-03 | |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
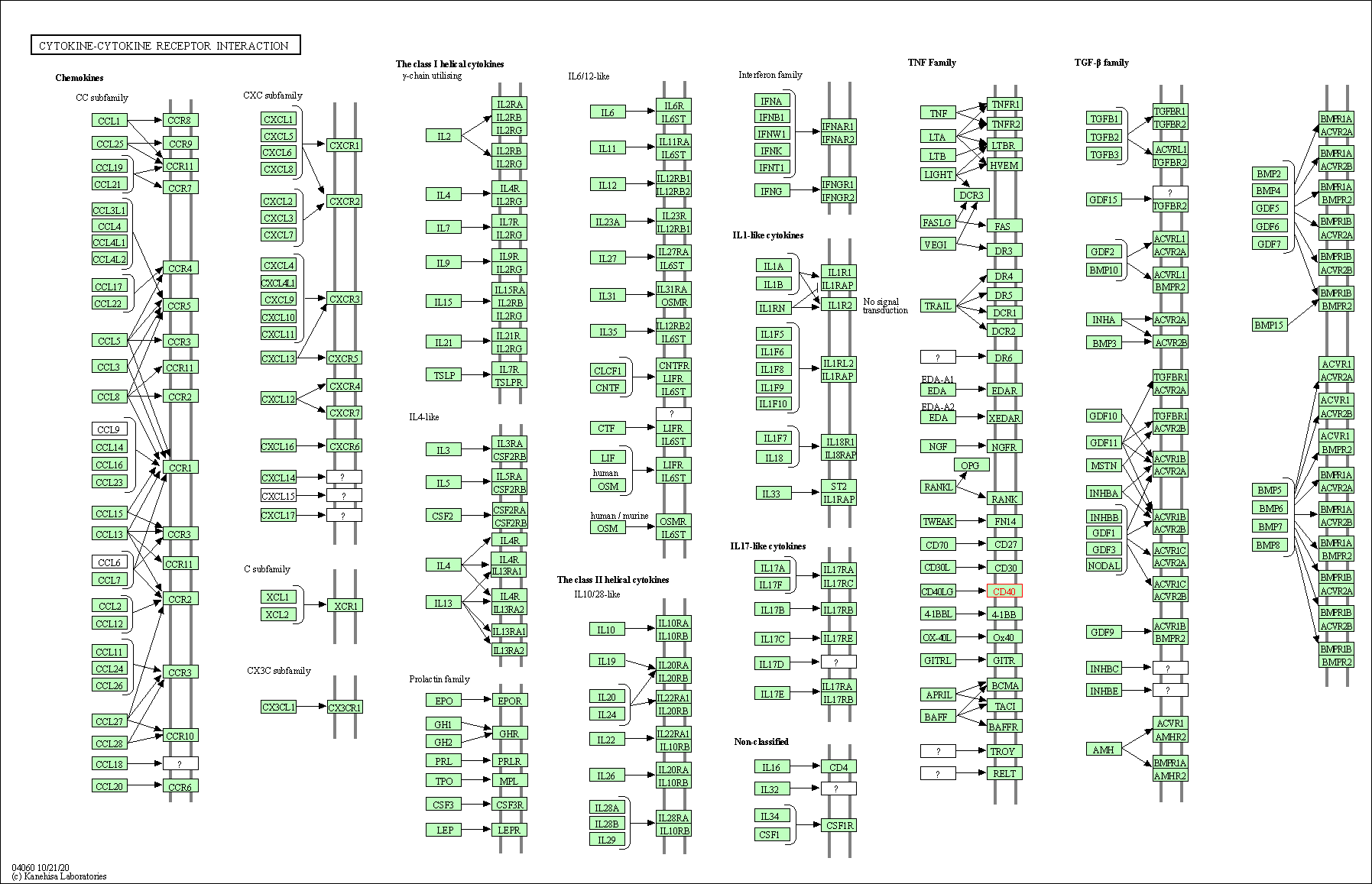
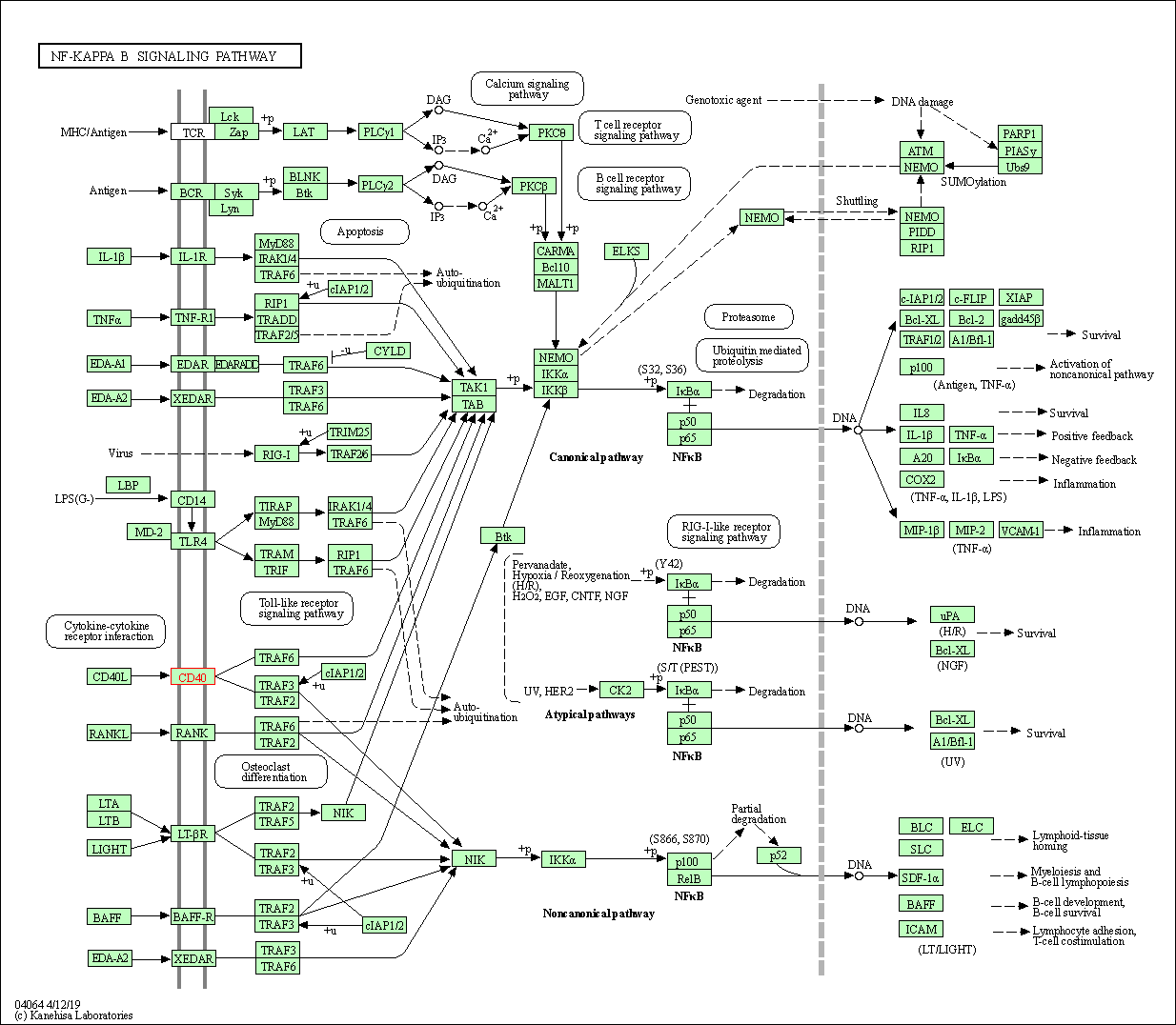
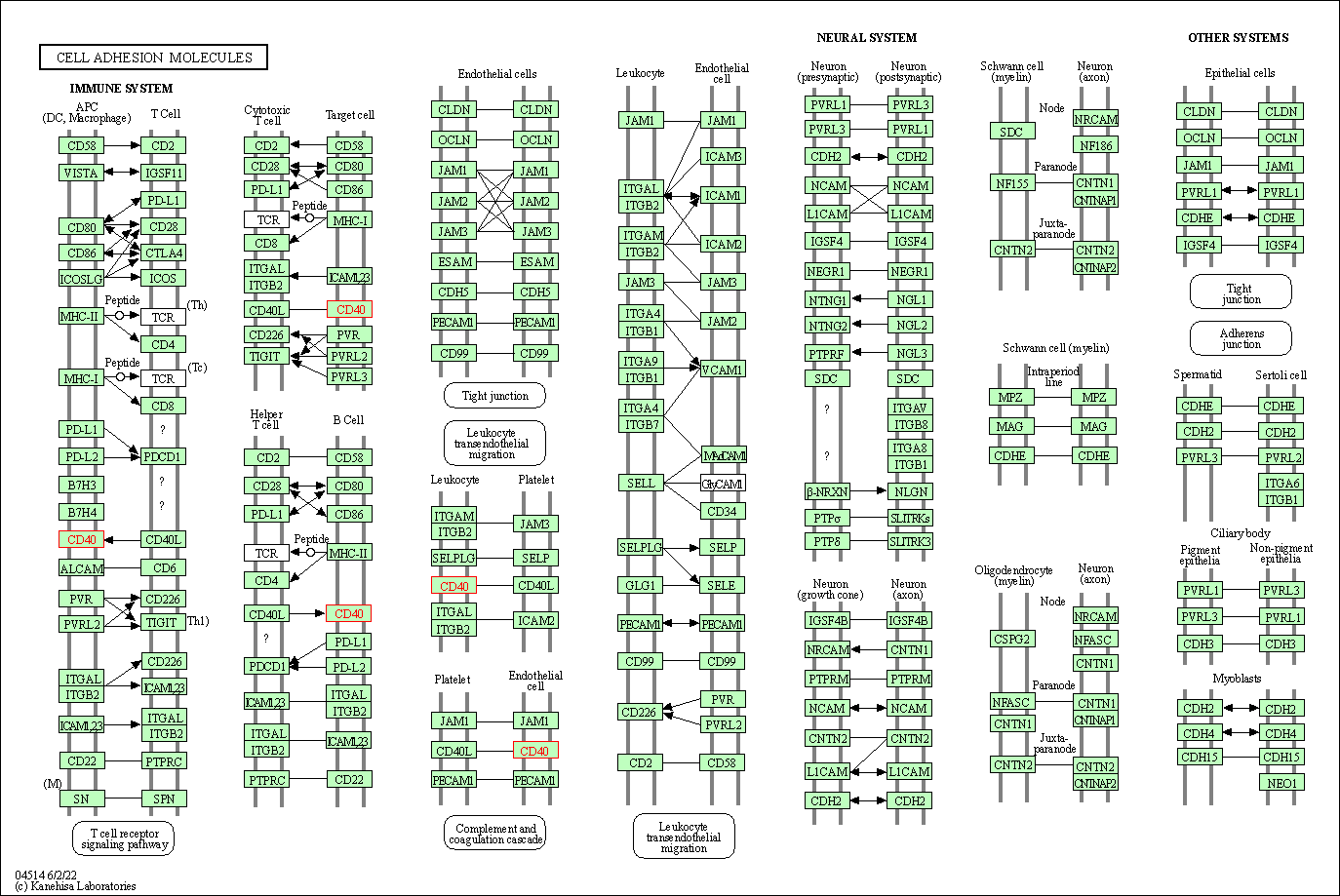
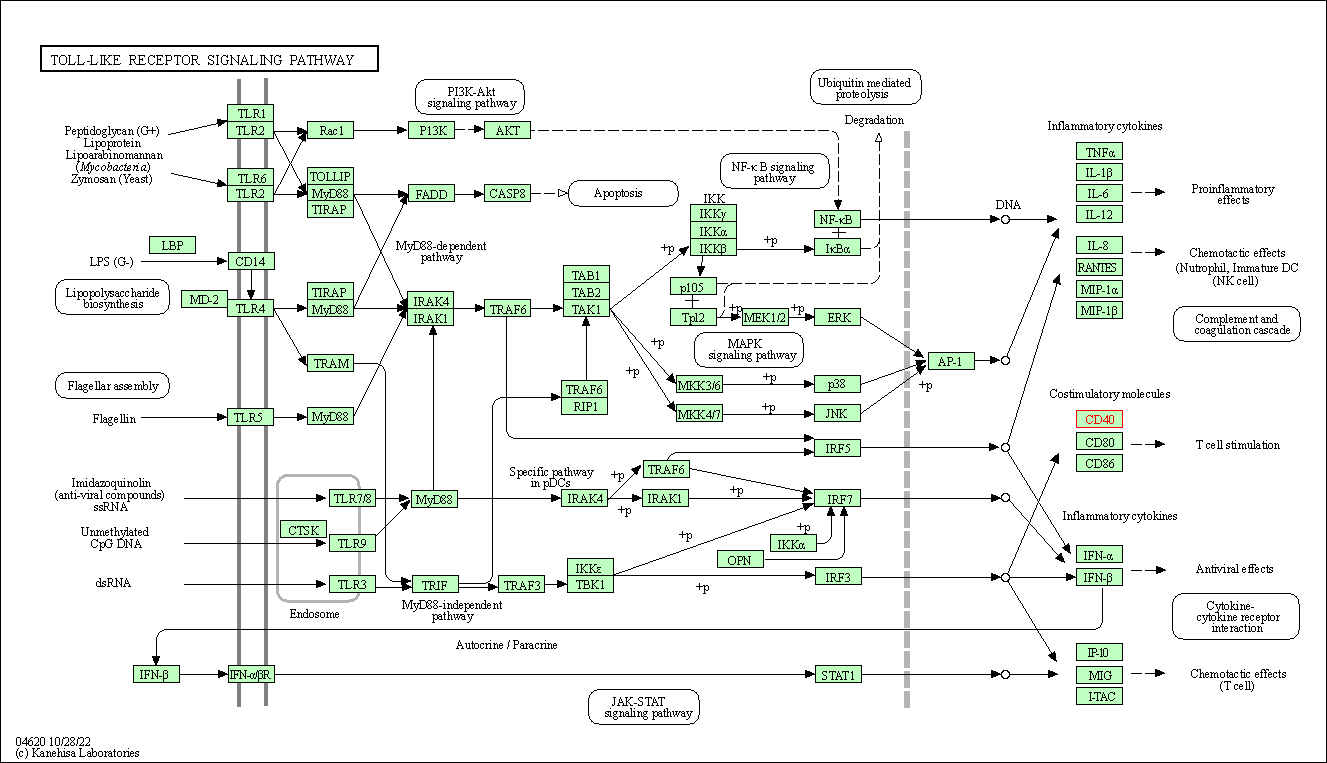
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cell adhesion molecules | hsa04514 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 29 | Degree centrality | 3.12E-03 | Betweenness centrality | 3.24E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.46E-01 | Radiality | 1.43E+01 | Clustering coefficient | 1.53E-01 |
| Neighborhood connectivity | 3.57E+01 | Topological coefficient | 6.27E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 16 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | NF-kappa B signaling pathway | |||||
| 3 | Cell adhesion molecules (CAMs) | |||||
| 4 | Toll-like receptor signaling pathway | |||||
| 5 | Intestinal immune network for IgA production | |||||
| 6 | Malaria | |||||
| 7 | Toxoplasmosis | |||||
| 8 | HTLV-I infection | |||||
| 9 | Epstein-Barr virus infection | |||||
| 10 | Transcriptional misregulation in cancer | |||||
| 11 | Asthma | |||||
| 12 | Autoimmune thyroid disease | |||||
| 13 | Systemic lupus erythematosus | |||||
| 14 | Allograft rejection | |||||
| 15 | Primary immunodeficiency | |||||
| 16 | Viral myocarditis | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | CD40/CD40L signaling | |||||
| WikiPathways | [+] 7 WikiPathways | + | ||||
| 1 | Toll-like receptor signaling pathway | |||||
| 2 | Inflammatory Response Pathway | |||||
| 3 | NLR Proteins | |||||
| 4 | Vitamin D Receptor Pathway | |||||
| 5 | Human Complement System | |||||
| 6 | Allograft Rejection | |||||
| 7 | Regulation of toll-like receptor signaling pathway | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1874). | |||||
| REF 2 | ClinicalTrials.gov (NCT04337931) A Study to Evaluate APX005M in Subjects With Unresectable or Metastatic Melanoma. U.S. National Institutes of Health. | |||||
| REF 3 | ClinicalTrials.gov (NCT01585233) A Multiple Dose Escalation Study of ASKP1240 in Subjects With Moderate to Severe Plaque Psoriasis. U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT04322149) A Phase 2a Open-Label, Multi-Center Study to Evaluate the Safety and Tolerability of Multiple Doses of AT-1501 in Adults With ALS. U.S.National Institutes of Health. | |||||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 6 | ClinicalTrials.gov (NCT02291029) Safety, Pharmacokinetics and Preliminary Efficacy Study of CFZ533 in Patients With Primary Sj ren's Syndrome. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT04046549) A Phase 2a Single-arm, Prospective, Open-label Pilot Study to Evaluate the Safety and Efficacy of Dual Costimulation Blockade With VIB4920 and Belatacept for Prophylaxis of Allograft Rejection in Adults Receiving a Kidney Transplant. U.S.National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT04879628) A Phase 2, Double-blind, Randomized, Placebo-controlled Study Assessing Efficacy and Safety of SAR441344, a CD40L-antagonist Monoclonal Antibody, in Participants With Relapsing Multiple Sclerosis. U.S.National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT04129528) Investigator- and Subject-blinded, Randomized, Placebo-controlled Study to Evaluate Safety, Tolerability, Pharmacokinetics and Efficacy Trial of CFZ533 in Pediatric and Young Adult Subjects With New Onset Type 1 Diabetes (T1DM). U.S.National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT05198310) A Phase 2, Multicenter, Randomized, Double-blind, Placebo Controlled Study to Assess the Safety, Pharmacokinetics, and Efficacy of KPL-404 in Subjects With Moderate to Severe Active Rheumatoid Arthritis With Inadequate Response or Intolerance to at Least One Biologic Disease-modifying Anti-rheumatic Drug or a Janus Kinase Inhibitor. U.S.National Institutes of Health. | |||||
| REF 11 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 12 | ClinicalTrials.gov (NCT01103635) Tremelimumab and CP-870,893 in Patients With Metastatic Melanoma. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT04083599) GEN1042 Safety Trial in Subjects With Malignant Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT03852511) First in Human Study of NG-350A (an Oncolytic Adenoviral Vector Which Expresses an Anti-CD40 Antibody) (FORTITUDE). U.S. National Institutes of Health. | |||||
| REF 15 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 16 | ClinicalTrials.gov (NCT04406623) Phase 1 Study of SL-172154 (SIRPalpha-Fc-CD40L) in Subjects With Ovarian Cancer. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT05017623) A Multicenter, Open-label, Phase 1 Dose Escalation Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of YH003 in Subjects With Advanced Solid Tumors. U.S.National Institutes of Health. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5077). | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008196) | |||||
| REF 20 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 21 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 22 | The enhanced immunopharmacology of VIB4920, a novel Tn3 fusion protein and CD40L antagonist, and assessment of its safety profile in cynomolgus monkeys. Br J Pharmacol. 2020 Mar;177(5):1061-1076. | |||||
| REF 23 | Developments in immunosuppression. Curr Opin Organ Transplant. 2021 Feb 1;26(1):91-96. | |||||
| REF 24 | Anti-CD40 antibody KPL-404 inhibits T cell-mediated activation of B cells from healthy donors and autoimmune patients. Arthritis Res Ther. 2021 Jan 6;23(1):5. | |||||
| REF 25 | Development of CDX-1140, an agonist CD40 antibody for cancer immunotherapy. Cancer Immunol Immunother. 2019 Feb;68(2):233-245. | |||||
| REF 26 | Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007 Mar 1;25(7):876-83. | |||||
| REF 27 | Clinical pipeline report, company report or official report of Genmab. | |||||
| REF 28 | Clinical pipeline report, company report or official report of PsiOxus Therapeutics. | |||||
| REF 29 | Clinical pipeline report, company report or official report of Shattuck Labs. | |||||
| REF 30 | Clinical pipeline report, company report or official report of Biocytogen | |||||
| REF 31 | Recombinant human CD40 ligand inhibits simian immunodeficiency virus replication: a role for interleukin- 16. J Med Primatol. 1999 Aug-Oct;28(4-5):190-4. | |||||
| REF 32 | Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 2001 Oct 15;61(20):7556-62. | |||||
| REF 33 | Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res. 2001 Mar;7(3):691-703. | |||||
| REF 34 | Clinical pipeline report, company report or official report of Biocytogen | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

