Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T62431
(Former ID: TTDC00156)
|
|||||
| Target Name |
Tyrosine-protein kinase SYK (SYK)
|
|||||
| Synonyms |
p72-Syk; Spleen tyrosine kinase
Click to Show/Hide
|
|||||
| Gene Name |
SYK
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Thrombocytopenia [ICD-11: 3B64] | |||||
| Function |
Regulates several biological processes including innate and adaptive immunity, cell adhesion, osteoclast maturation, platelet activation and vascular development. Assembles into signaling complexes with activated receptors at the plasma membrane via interaction between its SH2 domains and the receptor tyrosine-phosphorylated ITAM domains. The association with the receptor can also be indirect and mediated by adapter proteins containing ITAM or partial hemITAM domains. The phosphorylation of the ITAM domains is generally mediated by SRC subfamily kinases upon engagement of the receptor. More rarely signal transduction via SYK could be ITAM-independent. Direct downstream effectors phosphorylated by SYK include VAV1, PLCG1, PI-3-kinase, LCP2 and BLNK. Initially identified as essential in B-cell receptor (BCR) signaling, it is necessary for the maturation of B-cells most probably at the pro-B to pre-B transition. Activated upon BCR engagement, it phosphorylates and activates BLNK an adapter linking the activated BCR to downstream signaling adapters and effectors. It also phosphorylates and activates PLCG1 and the PKC signaling pathway. It also phosphorylates BTK and regulates its activity in B-cell antigen receptor (BCR)-coupled signaling. In addition to its function downstream of BCR plays also a role in T-cell receptor signaling. Plays also a crucial role in the innate immune response to fungal, bacterial and viral pathogens. It is for instance activated by the membrane lectin CLEC7A. Upon stimulation by fungal proteins, CLEC7A together with SYK activates immune cells inducing the production of ROS. Also activates the inflammasome and NF-kappa-B-mediated transcription of chemokines and cytokines in presence of pathogens. Regulates neutrophil degranulation and phagocytosis through activation of the MAPK signaling cascade. Required for the stimulation of neutrophil phagocytosis by IL15. Also mediates the activation of dendritic cells by cell necrosis stimuli. Also involved in mast cells activation. Involved in interleukin-3/IL3-mediated signaling pathway in basophils. Also functions downstream of receptors mediating cell adhesion. Relays for instance, integrin-mediated neutrophils and macrophages activation and P-selectin receptor/SELPG-mediated recruitment of leukocytes to inflammatory loci. Plays also a role in non-immune processes. It is for instance involved in vascular development where it may regulate blood and lymphatic vascular separation. It is also required for osteoclast development and function. Functions in the activation of platelets by collagen, mediating PLCG2 phosphorylation and activation. May be coupled to the collagen receptor by the ITAM domain-containing FCER1G. Also activated by the membrane lectin CLEC1B that is required for activation of platelets by PDPN/podoplanin. Involved in platelet adhesion being activated by ITGB3 engaged by fibrinogen. Together with CEACAM20, enhances production of the cytokine CXCL8/IL-8 via the NFKB pathway and may thus have a role in the intestinal immune response. Non-receptor tyrosine kinase which mediates signal transduction downstream of a variety of transmembrane receptors including classical immunoreceptors like the B-cell receptor (BCR).
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.2
|
|||||
| Sequence |
MASSGMADSANHLPFFFGNITREEAEDYLVQGGMSDGLYLLRQSRNYLGGFALSVAHGRK
AHHYTIERELNGTYAIAGGRTHASPADLCHYHSQESDGLVCLLKKPFNRPQGVQPKTGPF EDLKENLIREYVKQTWNLQGQALEQAIISQKPQLEKLIATTAHEKMPWFHGKISREESEQ IVLIGSKTNGKFLIRARDNNGSYALCLLHEGKVLHYRIDKDKTGKLSIPEGKKFDTLWQL VEHYSYKADGLLRVLTVPCQKIGTQGNVNFGGRPQLPGSHPATWSAGGIISRIKSYSFPK PGHRKSSPAQGNRQESTVSFNPYEPELAPWAADKGPQREALPMDTEVYESPYADPEEIRP KEVYLDRKLLTLEDKELGSGNFGTVKKGYYQMKKVVKTVAVKILKNEANDPALKDELLAE ANVMQQLDNPYIVRMIGICEAESWMLVMEMAELGPLNKYLQQNRHVKDKNIIELVHQVSM GMKYLEESNFVHRDLAARNVLLVTQHYAKISDFGLSKALRADENYYKAQTHGKWPVKWYA PECINYYKFSSKSDVWSFGVLMWEAFSYGQKPYRGMKGSEVTAMLEKGERMGCPAGCPRE MYDLMNLCWTYDVENRPGFAAVELRLRNYYYDVVN Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T69XEM | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Fostamatinib | Drug Info | Approved | Immune thrombocytopenic purpura | [2] | |
| Clinical Trial Drug(s) | [+] 16 Clinical Trial Drugs | + | ||||
| 1 | Fostamatinib disodium | Drug Info | Phase 3 | Thrombocytopenia | [3] | |
| 2 | Cerdulatinib | Drug Info | Phase 2 | B-cell lymphoma | [4] | |
| 3 | Cevidoplenib | Drug Info | Phase 2 | Rheumatoid arthritis | [5] | |
| 4 | GS-9876 | Drug Info | Phase 2 | Cutaneous lupus erythematosus | [6] | |
| 5 | GS-9973 | Drug Info | Phase 2 | B-cell lymphoma | [7], [8] | |
| 6 | TAK-659 | Drug Info | Phase 2 | Diffuse large B-cell lymphoma | [4] | |
| 7 | ALXN2075 | Drug Info | Phase 1/2 | Non-hodgkin lymphoma | [9] | |
| 8 | HM43239 | Drug Info | Phase 1/2 | Acute myeloid leukaemia | [10] | |
| 9 | BI 894416 | Drug Info | Phase 1 | Asthma | [11] | |
| 10 | GSK2646264 | Drug Info | Phase 1 | Cutaneous lupus erythematosus | [6] | |
| 11 | HMPL-523 | Drug Info | Phase 1 | Non-hodgkin lymphoma | [12] | |
| 12 | PRT-062607 | Drug Info | Phase 1 | Chronic lymphocytic leukaemia | [13], [14] | |
| 13 | PRT6207 | Drug Info | Phase 1 | Solid tumour/cancer | [15] | |
| 14 | PUR1800 | Drug Info | Phase 1 | Chronic obstructive pulmonary disease | [16] | |
| 15 | SKI-O-703 | Drug Info | Phase 1 | Rheumatoid arthritis | [17] | |
| 16 | tamatinib | Drug Info | Clinical trial | Solid tumour/cancer | [18] | |
| Discontinued Drug(s) | [+] 2 Discontinued Drugs | + | ||||
| 1 | R-343 | Drug Info | Discontinued in Phase 2 | Asthma | [19] | |
| 2 | PF-3526299 | Drug Info | Discontinued in Phase 1 | Asthma | [20] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | Fostamatinib | Drug Info | [21] | |||
| 2 | Cerdulatinib | Drug Info | [22] | |||
| 3 | GS-9973 | Drug Info | [24] | |||
| 4 | PRT6207 | Drug Info | [30] | |||
| Inhibitor | [+] 29 Inhibitor drugs | + | ||||
| 1 | Fostamatinib disodium | Drug Info | [3] | |||
| 2 | Cevidoplenib | Drug Info | [23] | |||
| 3 | GS-9876 | Drug Info | [6], [17] | |||
| 4 | TAK-659 | Drug Info | [25] | |||
| 5 | ALXN2075 | Drug Info | [26] | |||
| 6 | HM43239 | Drug Info | [27] | |||
| 7 | BI 894416 | Drug Info | [28] | |||
| 8 | GSK2646264 | Drug Info | [6] | |||
| 9 | HMPL-523 | Drug Info | [29] | |||
| 10 | PRT-062607 | Drug Info | [30] | |||
| 11 | PUR1800 | Drug Info | [31] | |||
| 12 | SKI-O-703 | Drug Info | [17] | |||
| 13 | tamatinib | Drug Info | [32] | |||
| 14 | Pyrimidopyridazinone derivative 1 | Drug Info | [33] | |||
| 15 | Pyrimidopyridazinone derivative 2 | Drug Info | [33] | |||
| 16 | Pyrrolo-pyrazine derivative 3 | Drug Info | [34] | |||
| 17 | Pyrrolo-pyrazine derivative 4 | Drug Info | [34] | |||
| 18 | R-343 | Drug Info | [35] | |||
| 19 | PF-3526299 | Drug Info | [36] | |||
| 20 | CG-103065 | Drug Info | [21] | |||
| 21 | DNX-2000 | Drug Info | [21] | |||
| 22 | ELLAGIC ACID | Drug Info | [37] | |||
| 23 | K00592a | Drug Info | [38] | |||
| 24 | N-Benzyl-4-(2,5-dihydroxy-benzylamino)-benzamide | Drug Info | [39] | |||
| 25 | PMID17600705C23 | Drug Info | [40] | |||
| 26 | PMID23312943C21 | Drug Info | [41] | |||
| 27 | PRT-060318 | Drug Info | [21] | |||
| 28 | SBB007833 | Drug Info | [42] | |||
| 29 | VRT-750018 | Drug Info | [21] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Imatinib | Ligand Info | |||||
| Structure Description | Crystal structure of the syk tyrosine kinase domain with Gleevec | PDB:1XBB | ||||
| Method | X-ray diffraction | Resolution | 1.57 Å | Mutation | No | [43] |
| PDB Sequence |
VYLDRKLLTL
372 EDKELGSGNF382 GTVKKGYYQM392 KKVVKTVAVK402 ILKPALKDEL417 LAEANVMQQL 427 DNPYIVRMIG437 ICEAESWMLV447 MEMAELGPLN457 KYLQQNRHVK467 DKNIIELVHQ 477 VSMGMKYLEE487 SNFVHRDLAA497 RNVLLVTQHY507 AKISDFGLSK517 ALRADENYYK 527 AKWPVKWYAP541 ECINYYKFSS551 KSDVWSFGVL561 MWEAFSYGQK571 PYRGMKGSEV 581 TAMLEKGERM591 GCPAGCPREM601 YDLMNLCWTY611 DVENRPGFAA621 VELRLRNYYY 631 DVVNEGHH
|
|||||
|
|
||||||
| Ligand Name: GS-9973 | Ligand Info | |||||
| Structure Description | Crystal structure of spleen tyrosine kinase (Syk) in complex with GS-9973 | PDB:4PUZ | ||||
| Method | X-ray diffraction | Resolution | 2.08 Å | Mutation | No | [44] |
| PDB Sequence |
VYLDRKLLTL
372 EDKELGSGNF382 GTVKKGYYQM392 KKVVKTVAVK402 ILKPALKDEL417 LAEANVMQQL 427 DNPYIVRMIG437 ICEAESWMLV447 MEMAELGPLN457 KYLQQNRHVK467 DKNIIELVHQ 477 VSMGMKYLEE487 SNFVHRDLAA497 RNVLLVTQHY507 AKISDFGLSK517 ALRADENYYK 527 AQKWPVKWYA540 PECINYYKFS550 SKSDVWSFGV560 LMWEAFSYGQ570 KPYRGMKGSE 580 VTAMLEKGER590 MGCPAGCPRE600 MYDLMNLCWT610 YDVENRPGFA620 AVELRLRNYY 630 YDV
|
|||||
|
|
LYS375
4.748
LEU377
3.577
GLY378
3.829
SER379
3.375
GLY380
4.450
PHE382
3.704
VAL385
3.804
ALA400
3.631
LYS402
4.234
VAL433
4.049
MET448
3.726
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
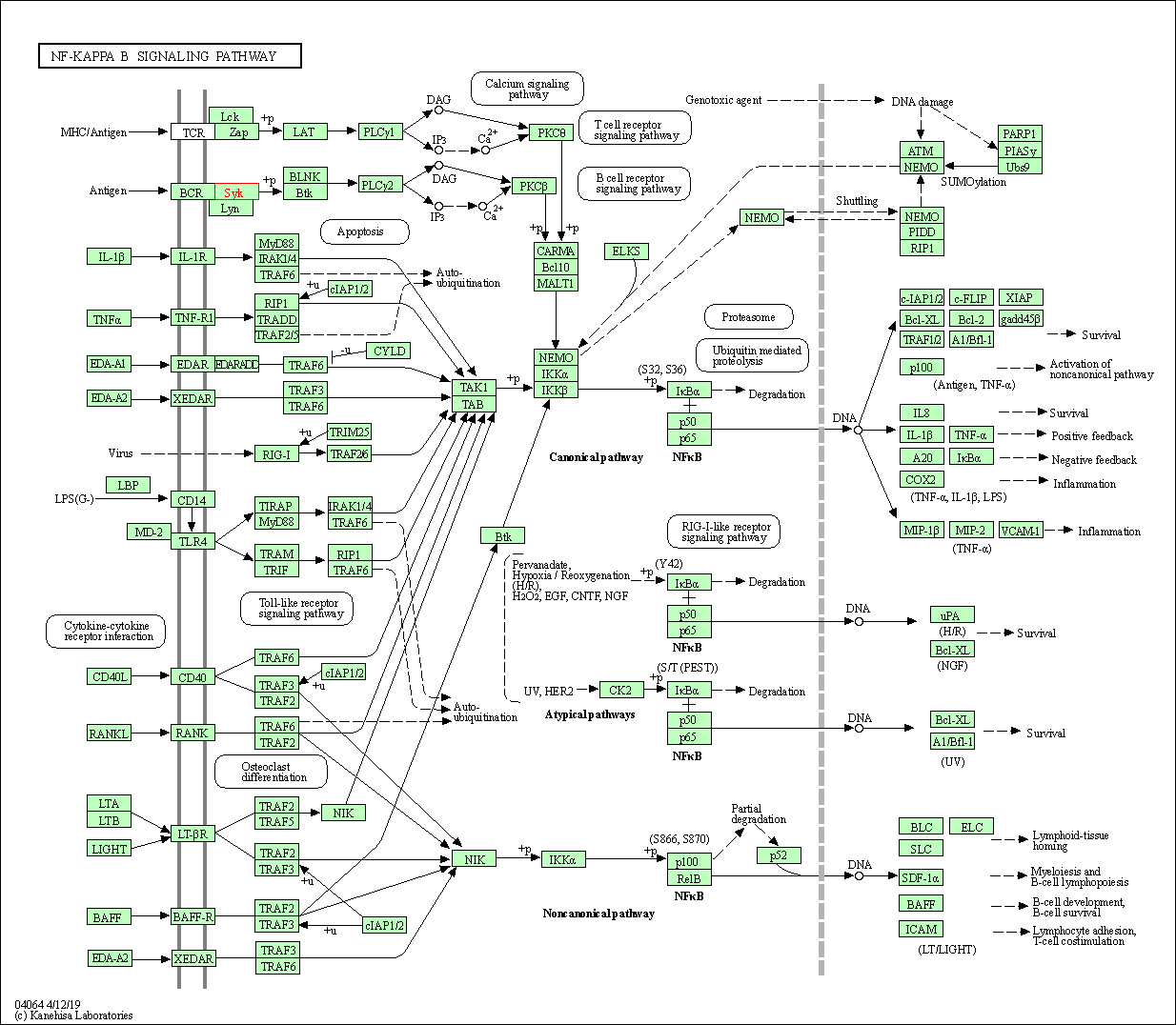
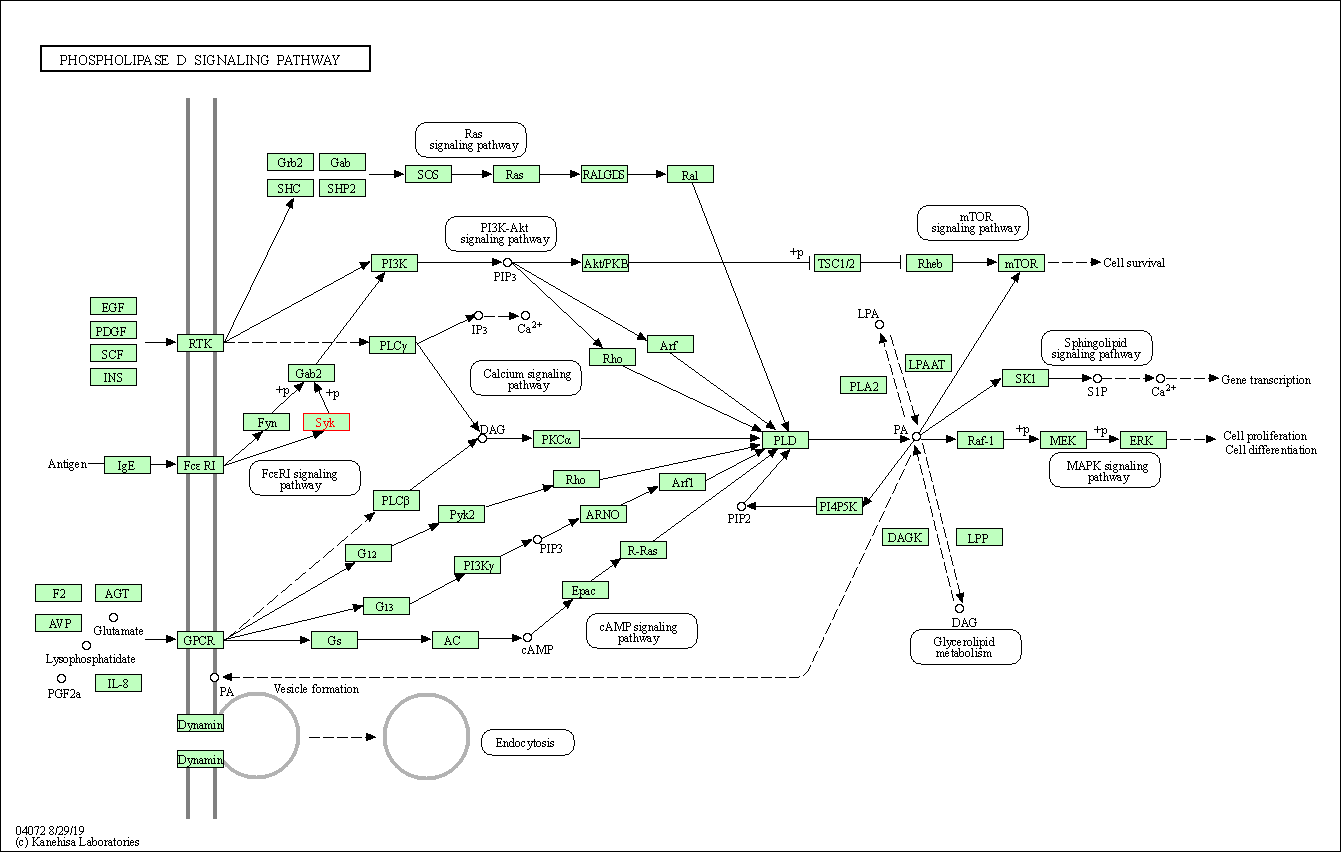
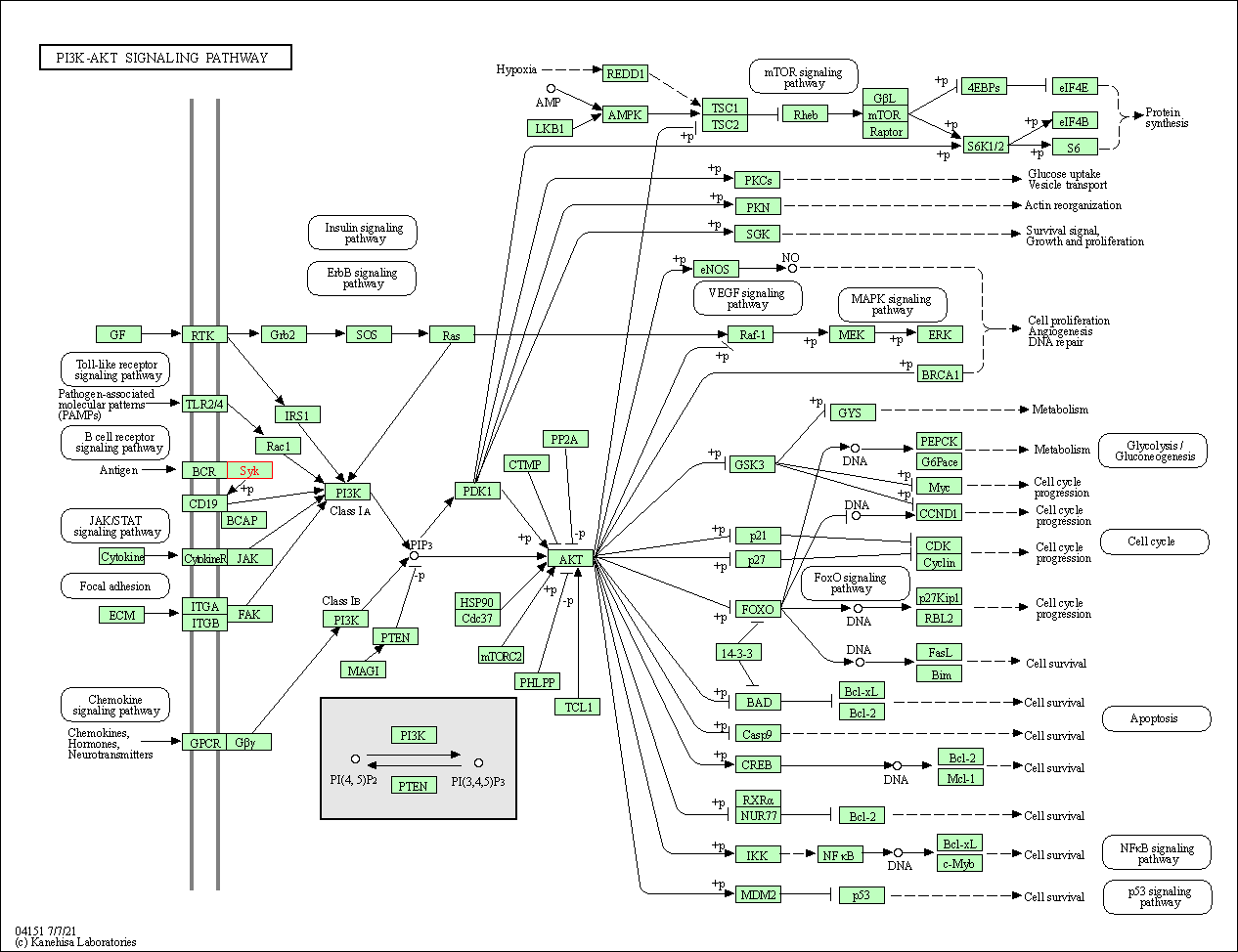
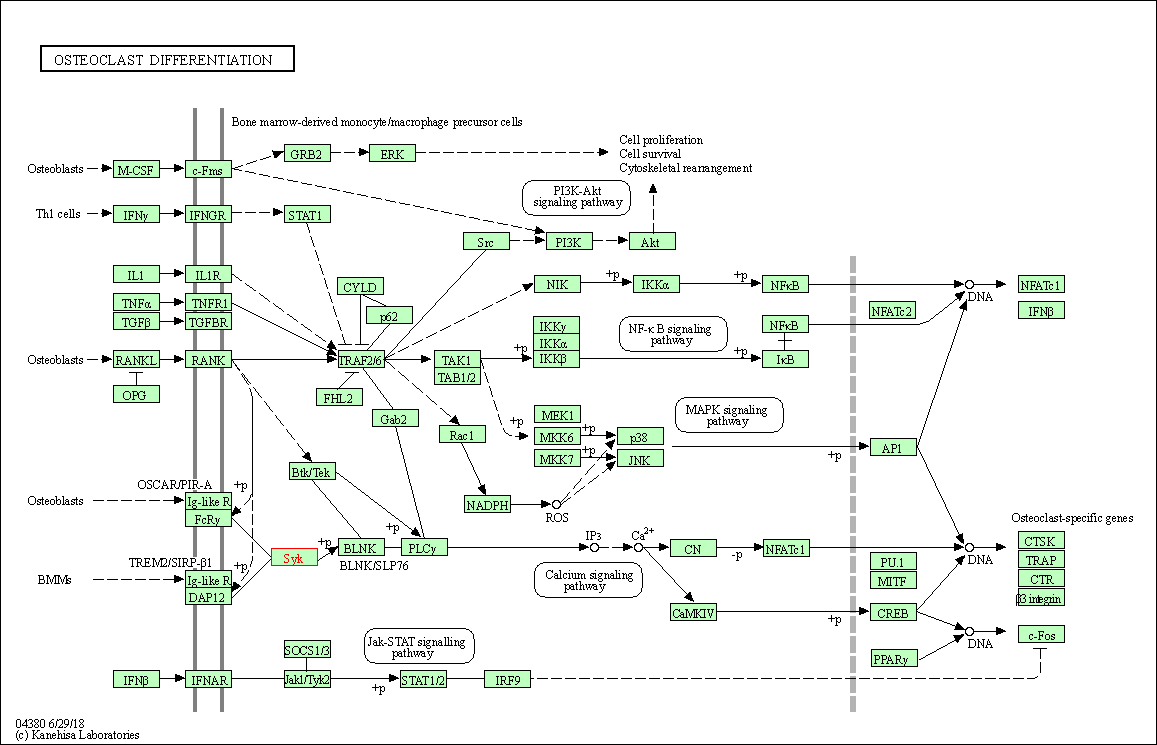
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Osteoclast differentiation | hsa04380 | Affiliated Target |

|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
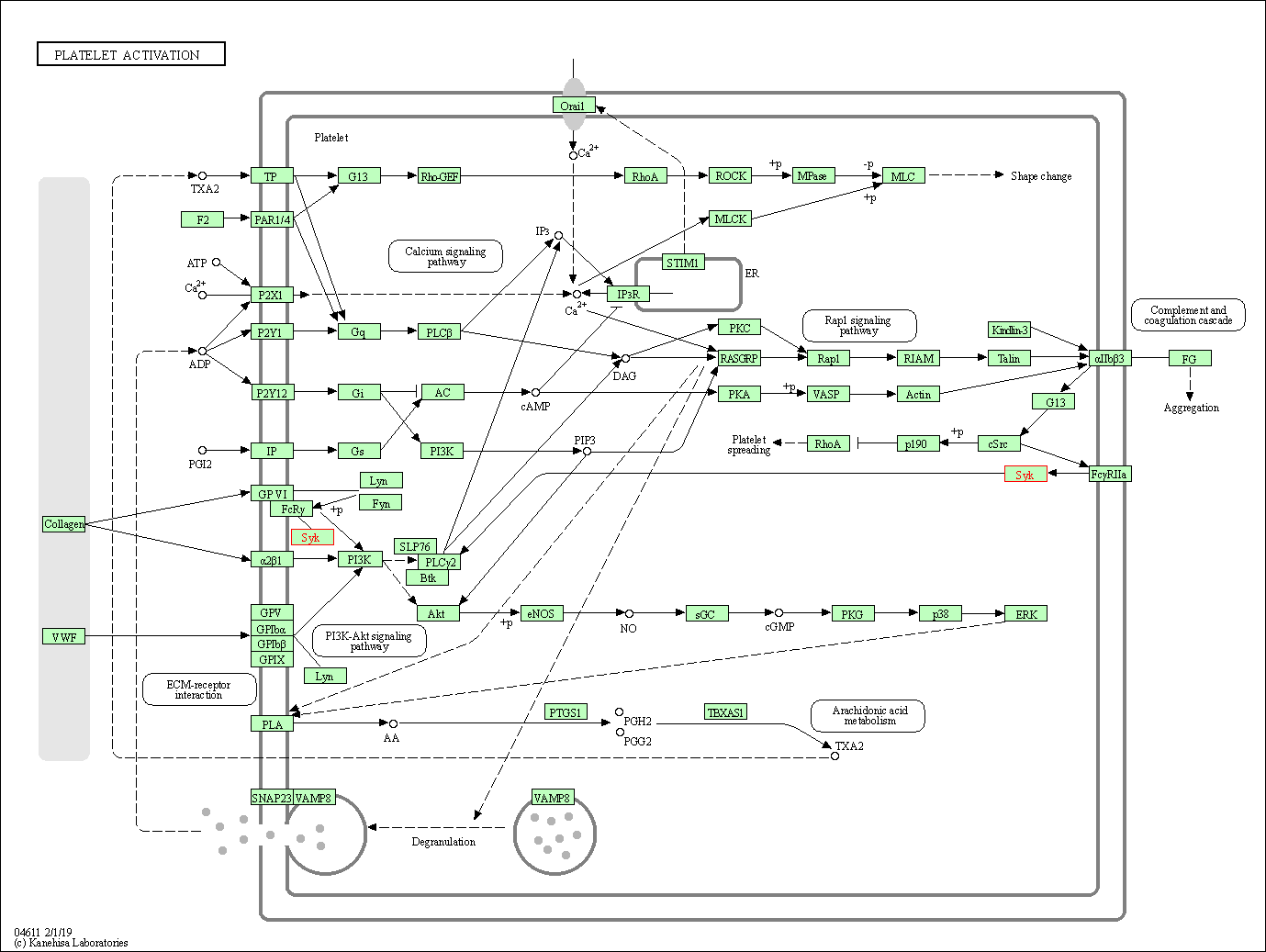
| Platelet activation | hsa04611 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
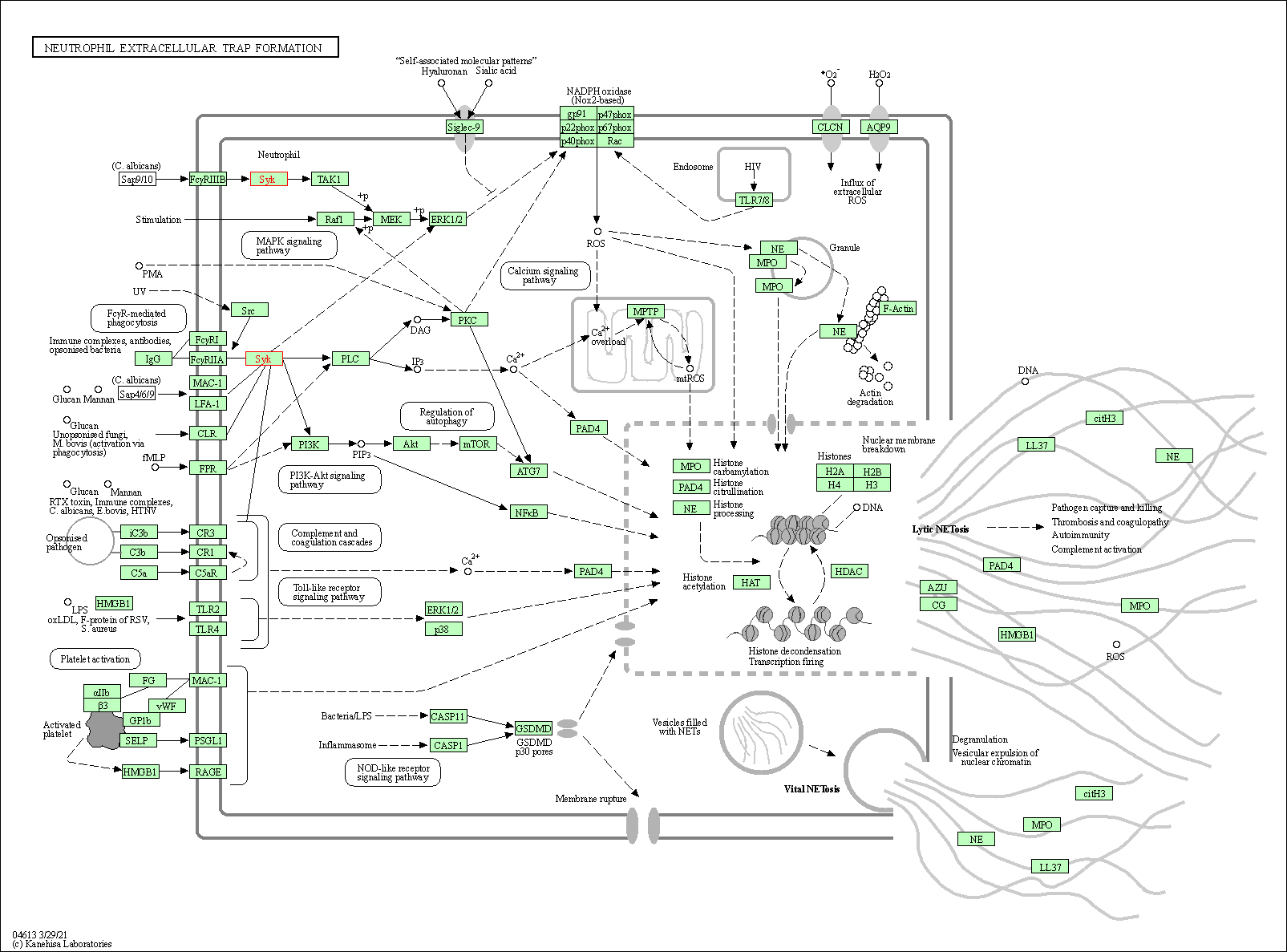
| Neutrophil extracellular trap formation | hsa04613 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
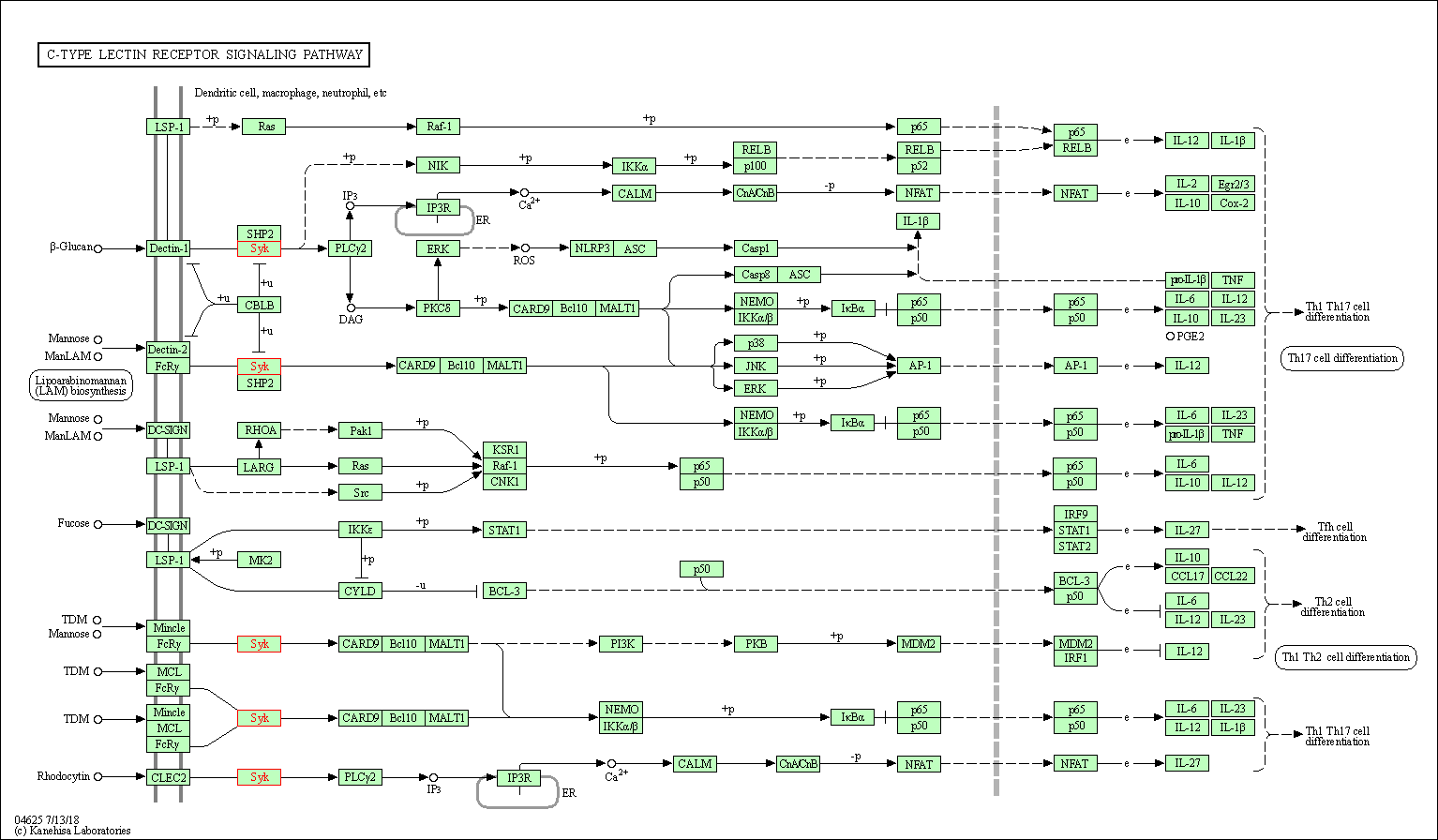
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
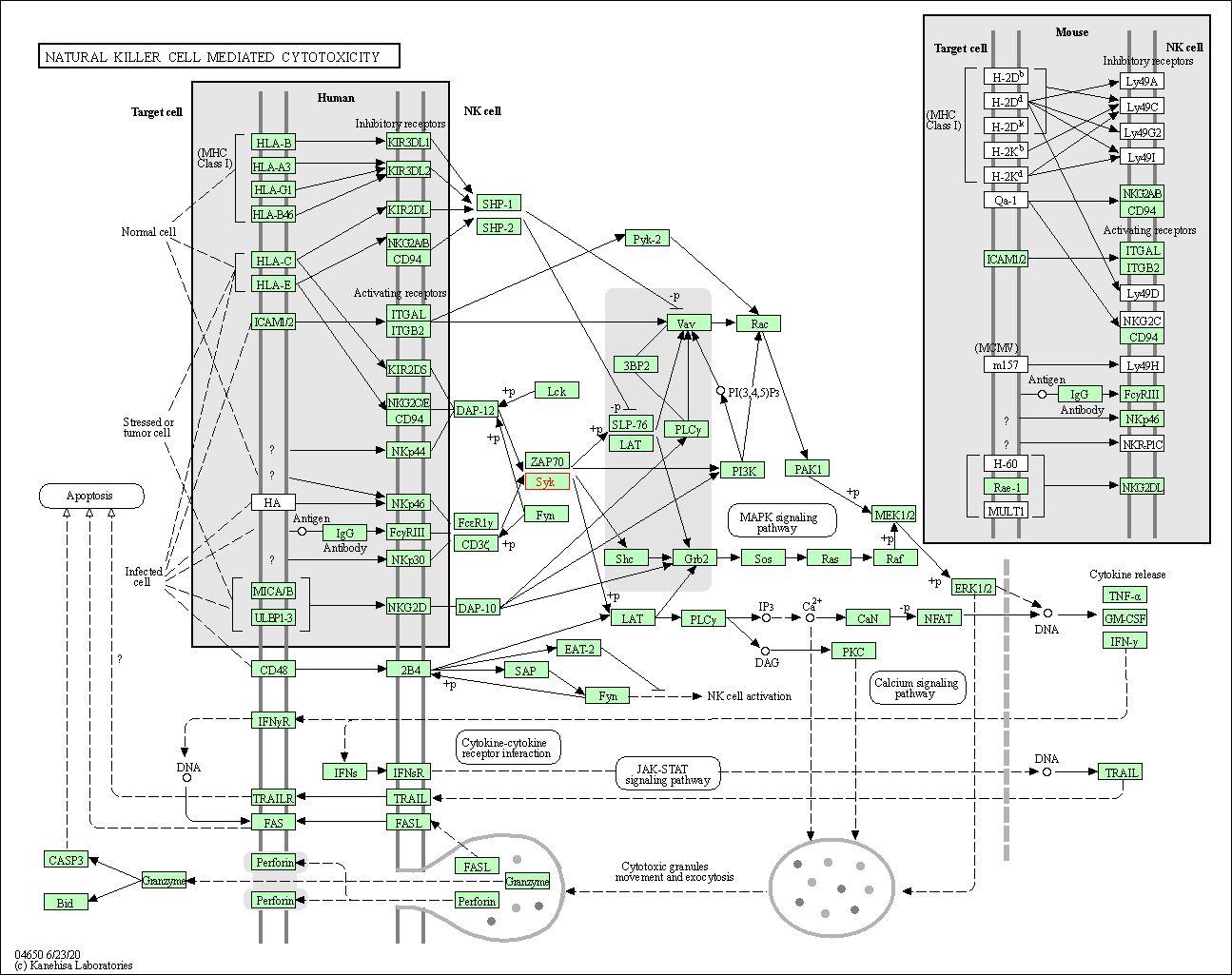
| Natural killer cell mediated cytotoxicity | hsa04650 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
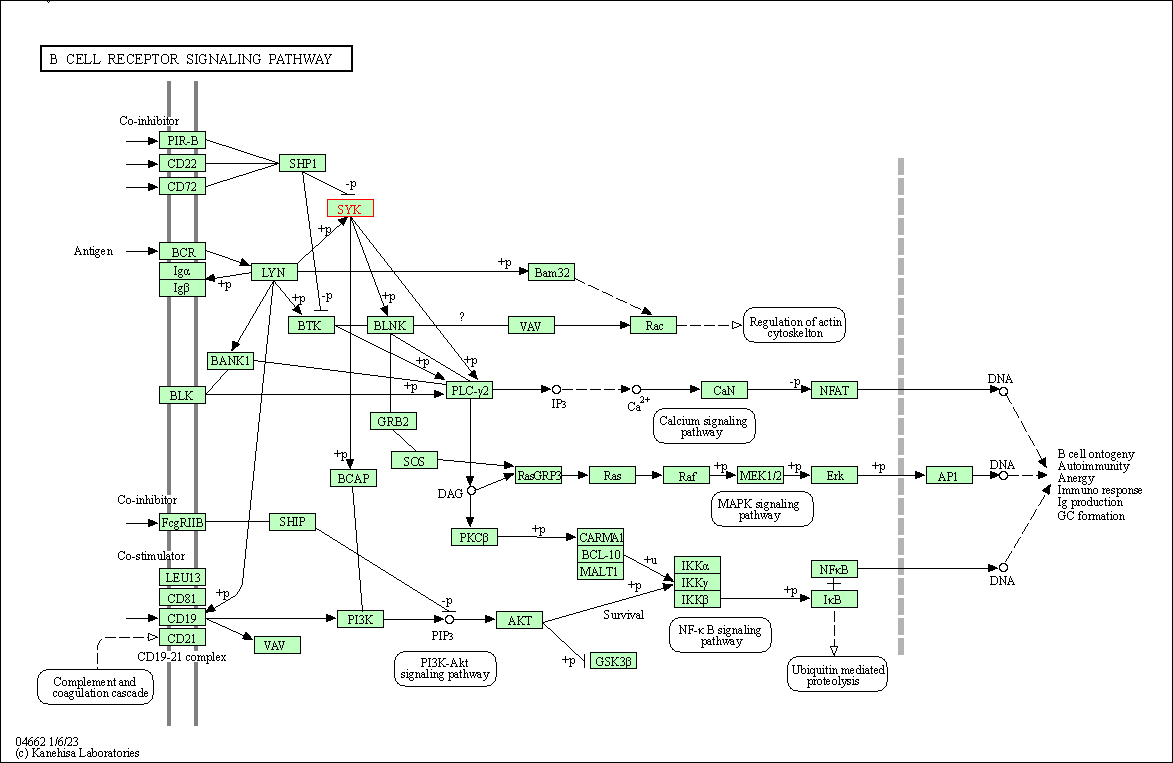
| B cell receptor signaling pathway | hsa04662 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
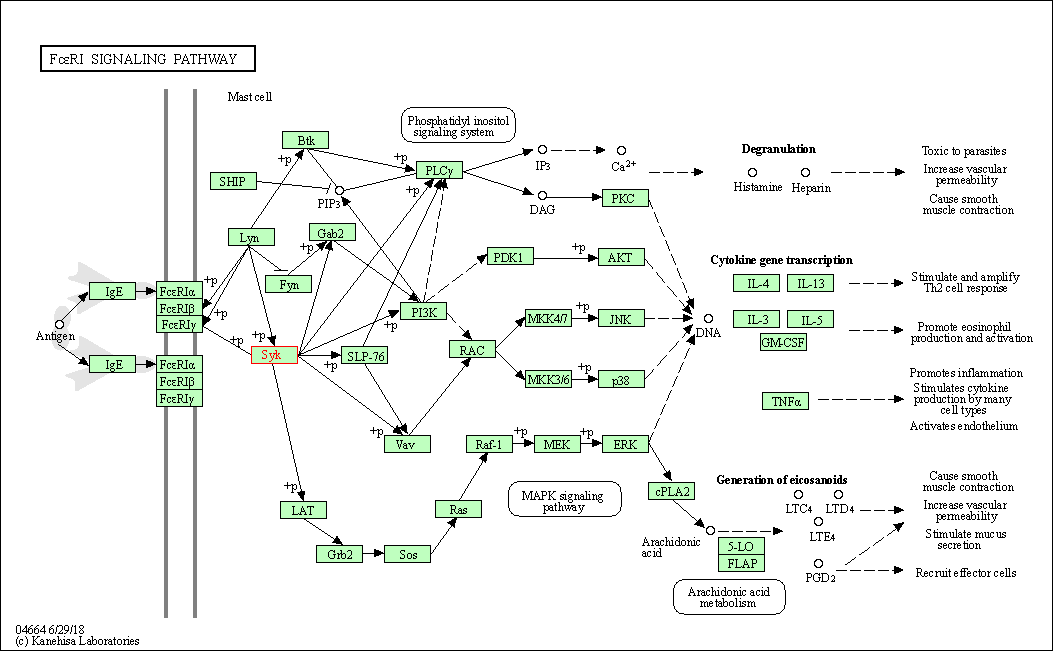
| Fc epsilon RI signaling pathway | hsa04664 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
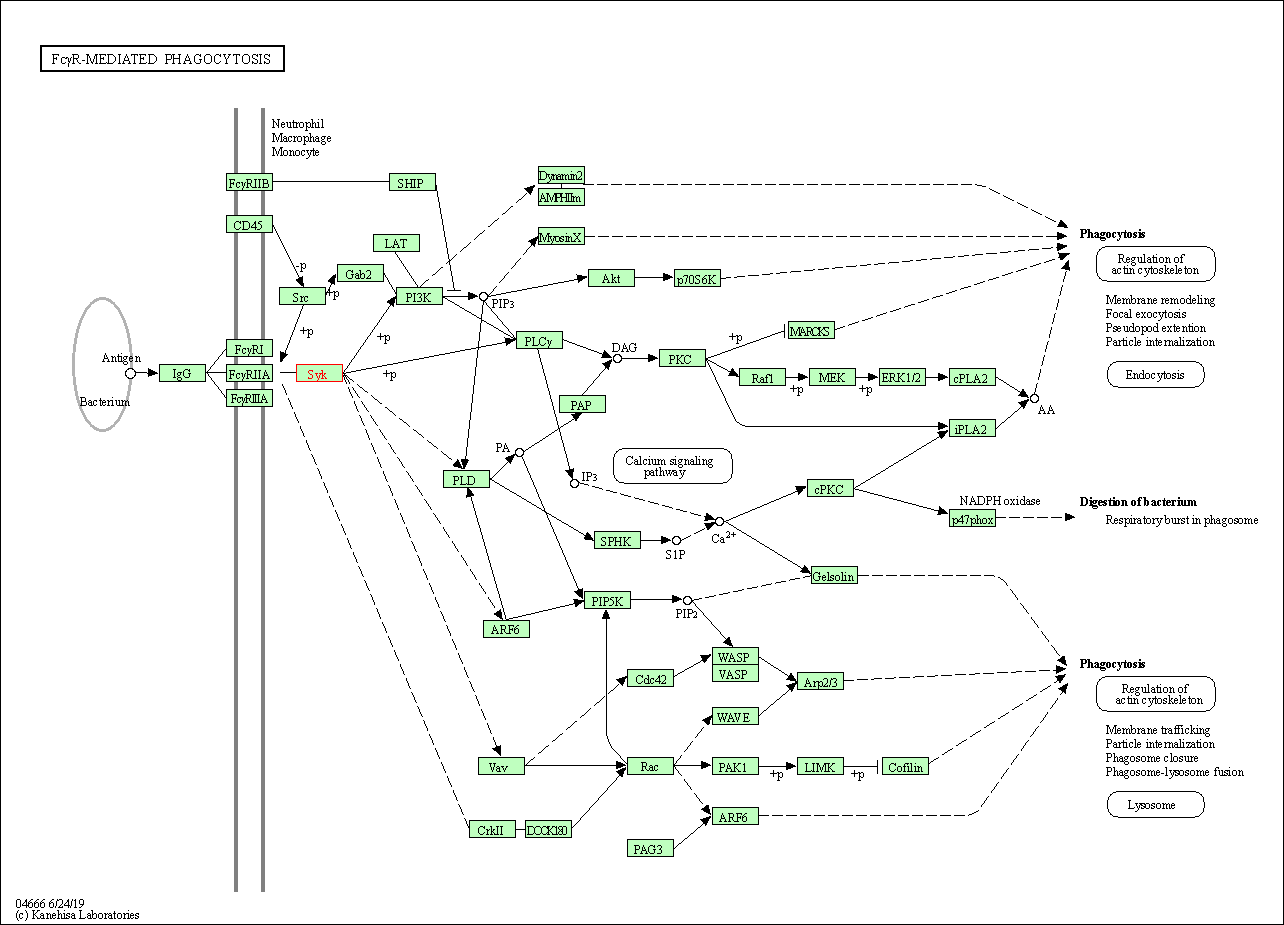
| Fc gamma R-mediated phagocytosis | hsa04666 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 57 | Degree centrality | 6.12E-03 | Betweenness centrality | 3.58E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.51E-01 | Radiality | 1.44E+01 | Clustering coefficient | 1.27E-01 |
| Neighborhood connectivity | 3.36E+01 | Topological coefficient | 4.62E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011 Jan;39(Database issue):D1035-41. | |||||
| REF 2 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 5 | Clinical pipeline report, company report or official report of Genosco | |||||
| REF 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7889). | |||||
| REF 8 | Clinical pipeline report, company report or official report of Gilead. | |||||
| REF 9 | ClinicalTrials.gov (NCT01994382) A Phase 1/2a Open-Label, Multi-Dose, Multi-Center Escalation and Exploratory Study of Cerdulatinib (PRT062070) in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) or B-Cell or T-Cell Non-Hodgkin Lymphoma (NHL). U.S.National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT03850574) Clinical Trial to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of HM43239 in Patients With Relapsed or Refractory Acute Myeloid Leukemia. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT03971539) Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single Rising Oral Doses and Multiple Rising Oral Doses of BI 894416 Versus Placebo in Male Patients With Asthma (Single-blind, Randomised, Placebo-controlled, Parallel Group Design). U.S.National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT03779113) An Open-label, Dose Escalation Trial to Evaluate the Safety and Pharmacokinetics of HMPL-523 in Patients With Lymphoma. U.S. National Institutes of Health. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8070). | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031836) | |||||
| REF 15 | More Than 900 Medicines and Vaccines in Clinical Testing Offer New Hope in the Fight Against Cancer. Pharmaceutical Research and Manufacturers of America report. 2012. | |||||
| REF 16 | ClinicalTrials.gov (NCT04759807) A Phase 1b 3-way Crossover Study to Assess the Safety, Tolerability and Pharmacokinetics of Repeated Once Daily Doses of PUR1800 in Adult Patients With Stable Chronic Obstructive Pulmonary Disease. U.S.National Institutes of Health. | |||||
| REF 17 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 18 | Developmental toxicity associated with receptor tyrosine kinase Ret inhibition in reproductive toxicity testing. Birth Defects Res A Clin Mol Teratol. 2009 Feb;85(2):130-6. | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027577) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027922) | |||||
| REF 21 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2230). | |||||
| REF 22 | Company report (Portola Pharmaceuticals) | |||||
| REF 23 | A novel selective spleen tyrosine kinase inhibitor SKI-O-703 (cevidoplenib) ameliorates lupus nephritis and serum-induced arthritis in murine models. Clin Exp Immunol. 2023 Mar 8;211(1):31-45. | |||||
| REF 24 | An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015 Apr 9;125(15):2336-43. | |||||
| REF 25 | J Clin Oncol 32:5s, 2014 (suppl; abstr 8580). | |||||
| REF 26 | The Dual Syk/JAK Inhibitor Cerdulatinib Antagonizes B-cell Receptor and Microenvironmental Signaling in Chronic Lymphocytic Leukemia. Clin Cancer Res. 2017 May 1;23(9):2313-2324. | |||||
| REF 27 | Clinical pipeline report, company report or official report of Hanmi Pharmaceutical. | |||||
| REF 28 | Synthesis of BI 894416 and BI 1342561, two potent and selective spleen tyrosine kinase inhibitors, labeled with carbon 14 and with deuterium. J Labelled Comp Radiopharm. 2023 Apr-May;66(4-6):155-168. | |||||
| REF 29 | Clinical pipeline report, company report or official report of Hutchison MediPharma. | |||||
| REF 30 | Advances in kinase inhibition: treating rheumatic diseases and beyond. Curr Opin Rheumatol. 2014 March; 26(2): 237-243. | |||||
| REF 31 | Clinical pipeline report, company report or official report of Pulmatrix Lexington, MA | |||||
| REF 32 | Pharmacophore modeling study based on known spleen tyrosine kinase inhibitors together with virtual screening for identifying novel inhibitors. Bioorg Med Chem Lett. 2009 Apr 1;19(7):1944-9. | |||||
| REF 33 | Inhibitors of JAK-family kinases: an update on the patent literature 2013-2015, part 1.Expert Opin Ther Pat. 2017 Feb;27(2):127-143. | |||||
| REF 34 | Inhibitors of JAK-family kinases: an update on the patent literature 2013-2015, part 2.Expert Opin Ther Pat. 2017 Feb;27(2):145-161. | |||||
| REF 35 | A novel Syk kinase inhibitor suitable for inhalation: R-343(. Expert Opin Ther Pat. 2009 Oct;19(10):1469-72. | |||||
| REF 36 | Pipeline report of RIGEL Pharmaceuticals. | |||||
| REF 37 | Identification of ellagic acid as potent inhibitor of protein kinase CK2: a successful example of a virtual screening application. J Med Chem. 2006 Apr 20;49(8):2363-6. | |||||
| REF 38 | Synthetic studies on novel Syk inhibitors. Part 1: Synthesis and structure-activity relationships of pyrimidine-5-carboxamide derivatives. Bioorg Med Chem. 2005 Aug 15;13(16):4936-51. | |||||
| REF 39 | Design, synthesis, and biological evaluation of a series of lavendustin A analogues that inhibit EGFR and Syk tyrosine kinases, as well as tubulin ... J Med Chem. 2001 Feb 1;44(3):441-52. | |||||
| REF 40 | N-4-Pyrimidinyl-1H-indazol-4-amine inhibitors of Lck: indazoles as phenol isosteres with improved pharmacokinetics. Bioorg Med Chem Lett. 2007 Aug 1;17(15):4363-8. | |||||
| REF 41 | Optimization of highly selective 2,4-diaminopyrimidine-5-carboxamide inhibitors of Sky kinase. Bioorg Med Chem Lett. 2013 Feb 15;23(4):1051-5. | |||||
| REF 42 | Synthesis and pharmacological evaluation of novel beta-nitrostyrene derivatives as tyrosine kinase inhibitors with potent antiplatelet activity. Biochem Pharmacol. 2007 Aug 15;74(4):601-11. | |||||
| REF 43 | A novel mode of Gleevec binding is revealed by the structure of spleen tyrosine kinase. J Biol Chem. 2004 Dec 31;279(53):55827-32. | |||||
| REF 44 | Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem. 2014 May 8;57(9):3856-73. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

