Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T73724
(Former ID: TTDR00164)
|
|||||
| Target Name |
Neuronal acetylcholine receptor beta-4 (CHRNB4)
|
|||||
| Synonyms |
CHRNB4; Beta-4 nAChR
Click to Show/Hide
|
|||||
| Gene Name |
CHRNB4
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Nicotine use disorder [ICD-11: 6C4A] | |||||
| Function |
After binding acetylcholine, the AChR responds by an extensive change in conformation that affects all subunits and leads to opening of an ion-conducting channel across the plasma membrane.
Click to Show/Hide
|
|||||
| BioChemical Class |
Neurotransmitter receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MRRAPSLVLFFLVALCGRGNCRVANAEEKLMDDLLNKTRYNNLIRPATSSSQLISIKLQL
SLAQLISVNEREQIMTTNVWLKQEWTDYRLTWNSSRYEGVNILRIPAKRIWLPDIVLYNN ADGTYEVSVYTNLIVRSNGSVLWLPPAIYKSACKIEVKYFPFDQQNCTLKFRSWTYDHTE IDMVLMTPTASMDDFTPSGEWDIVALPGRRTVNPQDPSYVDVTYDFIIKRKPLFYTINLI IPCVLTTLLAILVFYLPSDCGEKMTLCISVLLALTFFLLLISKIVPPTSLDVPLIGKYLM FTMVLVTFSIVTSVCVLNVHHRSPSTHTMAPWVKRCFLHKLPTFLFMKRPGPDSSPARAF PPSKSCVTKPEATATSTSPSNFYGNSMYFVNPASAASKSPAGSTPVAIPRDFWLRSSGRF RQDVQEALEGVSFIAQHMKNDDEDQSVVEDWKYVAMVVDRLFLWVFMFVCVLGTVGLFLP PLFQTHAASEGPYAAQRD Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 1 Clinical Trial Drugs | + | ||||
| 1 | CYTISINE | Drug Info | Phase 3 | Tobacco dependence | [2] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | SIB-1553A | Drug Info | Discontinued in Phase 2 | Alzheimer disease | [3] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 14 Inhibitor drugs | + | ||||
| 1 | CYTISINE | Drug Info | [1] | |||
| 2 | HOMOEPIBATIDINE | Drug Info | [5] | |||
| 3 | (2S,3S)-2-(m-Tolyl)-3,5,5-trimethylmorpholin-2-ol | Drug Info | [6] | |||
| 4 | (2S,3S)-2-Phenyl-3,5,5-trimethylmorpholin-2-ol | Drug Info | [6] | |||
| 5 | 15-nor-18-Methoxycornaridine | Drug Info | [7] | |||
| 6 | 18-Dimethylaminocoronaridine | Drug Info | [7] | |||
| 7 | 18-methoxycoronaridinate 2-Hydroxyethylamide | Drug Info | [7] | |||
| 8 | 18-methoxycoronaridinate 2-methoxyethylamide | Drug Info | [7] | |||
| 9 | 18-Methylaminocoronaridine | Drug Info | [7] | |||
| 10 | 2-Acetylaminoethyl 18-methoxycoronaridinate | Drug Info | [7] | |||
| 11 | 2-Hydroxyethyl 18-methoxycoronaridinate | Drug Info | [7] | |||
| 12 | 2-Methoxyethyl 18-methoxycoronaridinate | Drug Info | [7] | |||
| 13 | GCCSNPVCHLEHSNLC* | Drug Info | [8] | |||
| 14 | N,N-Dimethylaminoethyl 18-methoxycoronaridinate | Drug Info | [7] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | SIB-1553A | Drug Info | [4] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Nicotine | Ligand Info | |||||
| Structure Description | Human alpha3beta4 nicotinic acetylcholine receptor in complex with nicotine | PDB:6PV7 | ||||
| Method | Electron microscopy | Resolution | 3.34 Å | Mutation | No | [9] |
| PDB Sequence |
RVANAEEKLM
10 DDLLNKTRYN20 NLIRPATSSS30 QLISIKLQLS40 LAQLISVNER50 EQIMTTNVWL 60 KQEWTDYRLT70 WNSSRYEGVN80 ILRIPAKRIW90 LPDIVLYNNA100 DGTYEVSVYT 110 NLIVRSNGSV120 LWLPPAIYKS130 ACKIEVKYFP140 FDQQNCTLKF150 RSWTYDHTEI 160 DMVLMTPTAS170 MDDFTPSGEW180 DIVALPGRRT190 VNPQDPSYVD200 VTYDFIIKRK 210 PLFYTINLII220 PCVLTTLLAI230 LVFYLPSDCG240 EKMTLCISVL250 LALTFFLLLI 260 SKIVPPTSLD270 VPLIGKYLMF280 TMVLVTFSIV290 TSVCVLNVHH300 RSPSTHTMAP 310 WVKRCFLHKL320 PTFLFMKRRQ401 DVQEALEGVS411 FIAQHMKNDD421 EDQSVVEDWK 431 YVAMVVDRLF441 LWVFMFVCVL451 GTVGLFLP
|
|||||
|
|
||||||
| Ligand Name: Cholesterol hemisuccinate | Ligand Info | |||||
| Structure Description | Human alpha3beta4 nicotinic acetylcholine receptor in complex with nicotine | PDB:6PV7 | ||||
| Method | Electron microscopy | Resolution | 3.34 Å | Mutation | No | [9] |
| PDB Sequence |
RVANAEEKLM
10 DDLLNKTRYN20 NLIRPATSSS30 QLISIKLQLS40 LAQLISVNER50 EQIMTTNVWL 60 KQEWTDYRLT70 WNSSRYEGVN80 ILRIPAKRIW90 LPDIVLYNNA100 DGTYEVSVYT 110 NLIVRSNGSV120 LWLPPAIYKS130 ACKIEVKYFP140 FDQQNCTLKF150 RSWTYDHTEI 160 DMVLMTPTAS170 MDDFTPSGEW180 DIVALPGRRT190 VNPQDPSYVD200 VTYDFIIKRK 210 PLFYTINLII220 PCVLTTLLAI230 LVFYLPSDCG240 EKMTLCISVL250 LALTFFLLLI 260 SKIVPPTSLD270 VPLIGKYLMF280 TMVLVTFSIV290 TSVCVLNVHH300 RSPSTHTMAP 310 WVKRCFLHKL320 PTFLFMKRRQ401 DVQEALEGVS411 FIAQHMKNDD421 EDQSVVEDWK 431 YVAMVVDRLF441 LWVFMFVCVL451 GTVGLFLP
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
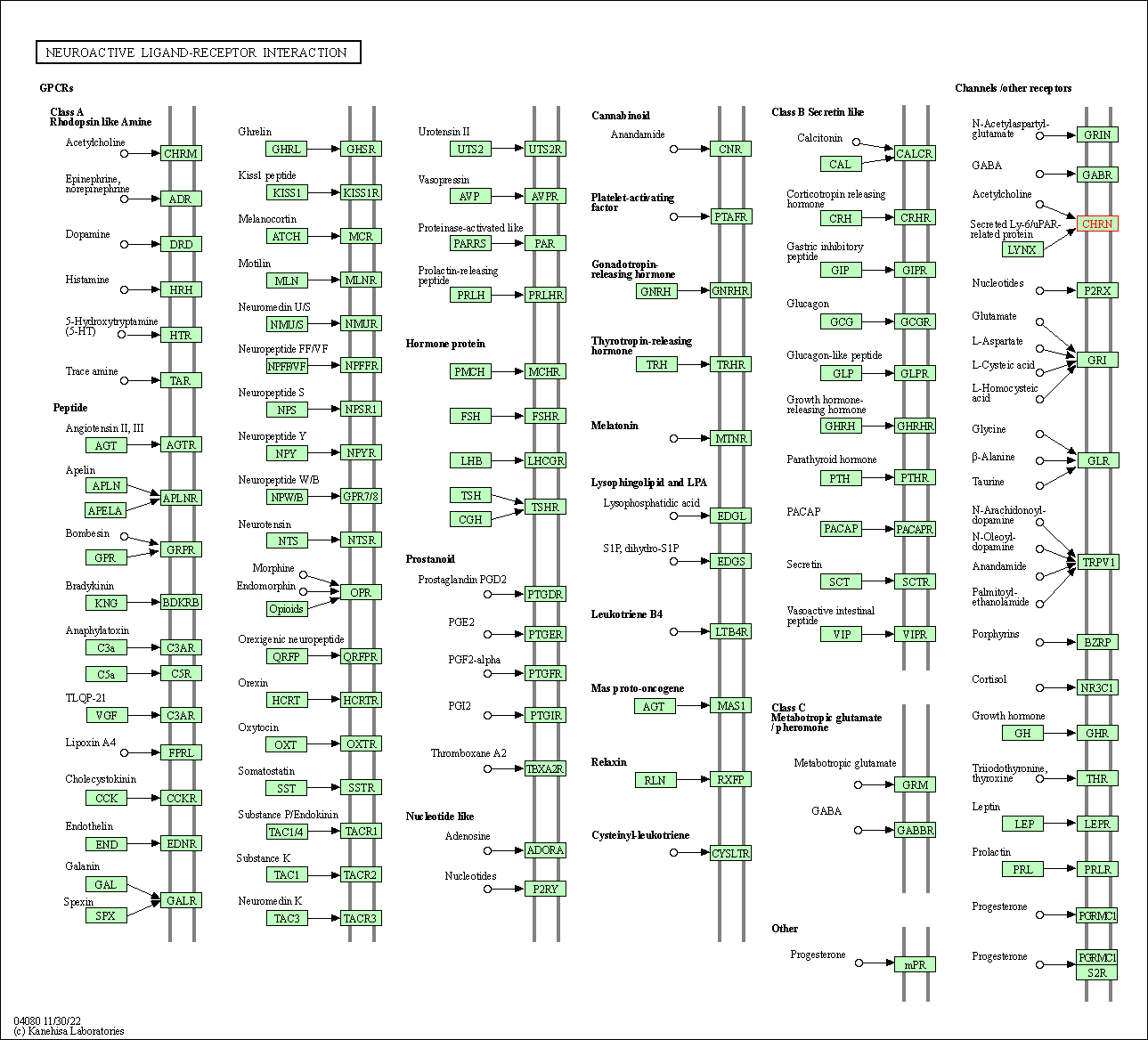
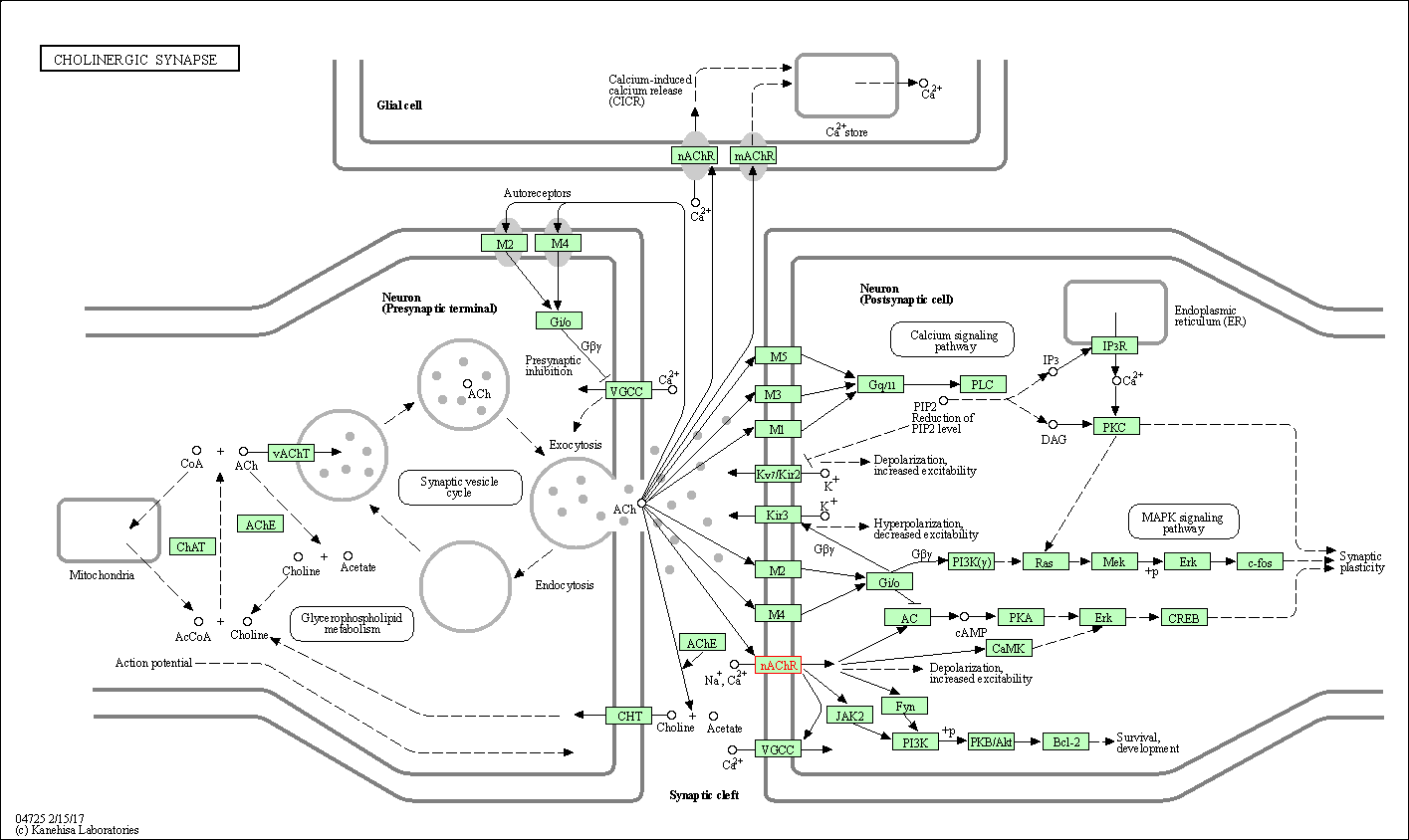
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Cholinergic synapse | hsa04725 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Neuroactive ligand-receptor interaction | |||||
| 2 | Cholinergic synapse | |||||
| Panther Pathway | [+] 2 Panther Pathways | + | ||||
| 1 | Nicotinic acetylcholine receptor signaling pathway | |||||
| 2 | Nicotine pharmacodynamics pathway | |||||
| Reactome | [+] 3 Reactome Pathways | + | ||||
| 1 | Highly sodium permeable acetylcholine nicotinic receptors | |||||
| 2 | Highly calcium permeable postsynaptic nicotinic acetylcholine receptors | |||||
| 3 | Highly calcium permeable nicotinic acetylcholine receptors | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | SIDS Susceptibility Pathways | |||||
| 2 | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| 3 | Nicotine Activity on Chromaffin Cells | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Deconstructing cytisine: The syntheses of (+/-)-cyfusine and (+/-)-cyclopropylcyfusine, fused ring analogs of cytisine. Bioorg Med Chem Lett. 2008 Apr 1;18(7):2316-9. | |||||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009631) | |||||
| REF 4 | SIB-1553A, (+/-)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride, a subtype-selective ligand for nicotinic acetylcholine receptors with putative cognitive-enhancing properties: effectson working and reference memory performances in aged rodents and nonhuman primates. J Pharmacol Exp Ther. 2001 Oct;299(1):297-306. | |||||
| REF 5 | Epibatidine isomers and analogues: structure-activity relationships. Bioorg Med Chem Lett. 2006 Nov 1;16(21):5493-7. | |||||
| REF 6 | Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: aids to smoking cessation. J Med Chem. 2010 Jun 24;53(12):4731-48. | |||||
| REF 7 | Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents. J Med Chem. 2003 Jun 19;46(13):2716-30. | |||||
| REF 8 | Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005 Jul 28;48(15):4705-45. | |||||
| REF 9 | Agonist Selectivity and Ion Permeation in the Alpha3beta4 Ganglionic Nicotinic Receptor. Neuron. 2019 Nov 6;104(3):501-511.e6. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

