Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T59328
(Former ID: TTDS00355)
|
|||||
| Target Name |
Epidermal growth factor receptor (EGFR)
|
|||||
| Synonyms |
Receptor tyrosine-protein kinase erbB-1; Proto-oncogene c-ErbB-1; HER1; ERBB1; ERBB
Click to Show/Hide
|
|||||
| Gene Name |
EGFR
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 10 Target-related Diseases | + | ||||
| 1 | Angina pectoris [ICD-11: BA40] | |||||
| 2 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| 3 | Colorectal cancer [ICD-11: 2B91] | |||||
| 4 | Diabetic foot ulcer [ICD-11: BD54] | |||||
| 5 | Ischemia [ICD-11: 8B10-8B11] | |||||
| 6 | Lung cancer [ICD-11: 2C25] | |||||
| 7 | Non-small-cell lung cancer [ICD-11: 2C25] | |||||
| 8 | Renal cell carcinoma [ICD-11: 2C90] | |||||
| 9 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 10 | Unspecific body region injury [ICD-11: ND56] | |||||
| Function |
Receptor tyrosine kinase binding ligands of the EGF family and activating several signaling cascades to convert extracellular cues into appropriate cellular responses. Known ligands include EGF, TGFA/TGF-alpha, AREG, epigen/EPGN, BTC/betacellulin, epiregulin/EREG and HBEGF/heparin-binding EGF. Ligand binding triggers receptor homo- and/or heterodimerization and autophosphorylation on key cytoplasmic residues. The phosphorylated receptor recruits adapter proteins like GRB2 which in turn activates complex downstream signaling cascades. Activates at least 4 major downstream signaling cascades including the RAS-RAF-MEK-ERK, PI3 kinase-AKT, PLCgamma-PKC and STATs modules. May also activate the NF-kappa-B signaling cascade. Also directly phosphorylates other proteins like RGS16, activating its GTPase activity and probably coupling the EGF receptor signaling to the G protein-coupled receptor signaling. Also phosphorylates MUC1 and increases its interaction with SRC and CTNNB1/beta-catenin. Plays a role in enhancing learning and memory performance (By similarity).
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MRPSGTAGAALLALLAALCPASRALEEKKVCQGTSNKLTQLGTFEDHFLSLQRMFNNCEV
VLGNLEITYVQRNYDLSFLKTIQEVAGYVLIALNTVERIPLENLQIIRGNMYYENSYALA VLSNYDANKTGLKELPMRNLQEILHGAVRFSNNPALCNVESIQWRDIVSSDFLSNMSMDF QNHLGSCQKCDPSCPNGSCWGAGEENCQKLTKIICAQQCSGRCRGKSPSDCCHNQCAAGC TGPRESDCLVCRKFRDEATCKDTCPPLMLYNPTTYQMDVNPEGKYSFGATCVKKCPRNYV VTDHGSCVRACGADSYEMEEDGVRKCKKCEGPCRKVCNGIGIGEFKDSLSINATNIKHFK NCTSISGDLHILPVAFRGDSFTHTPPLDPQELDILKTVKEITGFLLIQAWPENRTDLHAF ENLEIIRGRTKQHGQFSLAVVSLNITSLGLRSLKEISDGDVIISGNKNLCYANTINWKKL FGTSGQKTKIISNRGENSCKATGQVCHALCSPEGCWGPEPRDCVSCRNVSRGRECVDKCN LLEGEPREFVENSECIQCHPECLPQAMNITCTGRGPDNCIQCAHYIDGPHCVKTCPAGVM GENNTLVWKYADAGHVCHLCHPNCTYGCTGPGLEGCPTNGPKIPSIATGMVGALLLLLVV ALGIGLFMRRRHIVRKRTLRRLLQERELVEPLTPSGEAPNQALLRILKETEFKKIKVLGS GAFGTVYKGLWIPEGEKVKIPVAIKELREATSPKANKEILDEAYVMASVDNPHVCRLLGI CLTSTVQLITQLMPFGCLLDYVREHKDNIGSQYLLNWCVQIAKGMNYLEDRRLVHRDLAA RNVLVKTPQHVKITDFGLAKLLGAEEKEYHAEGGKVPIKWMALESILHRIYTHQSDVWSY GVTVWELMTFGSKPYDGIPASEISSILEKGERLPQPPICTIDVYMIMVKCWMIDADSRPK FRELIIEFSKMARDPQRYLVIQGDERMHLPSPTDSNFYRALMDEEDMDDVVDADEYLIPQ QGFFSSPSTSRTPLLSSLSATSNNSTVACIDRNGLQSCPIKEDSFLQRYSSDPTGALTED SIDDTFLPVPEYINQSVPKRPAGSVQNPVYHNQPLNPAPSRDPHYQDPHSTAVGNPEYLN TVQPTCVNSTFDSPAHWAQKGSHQISLDNPDYQQDFFPKEAKPNGIFKGSTAENAEYLRV APQSSEFIGA Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A00924 ; BADD_A01146 ; BADD_A01626 ; BADD_A02005 ; BADD_A05675 ; BADD_A06535 | |||||
| HIT2.0 ID | T43N2D | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 19 Approved Drugs | + | ||||
| 1 | Amivantamab | Drug Info | Approved | Non-small-cell lung cancer | [2] | |
| 2 | BIBW 2992 | Drug Info | Approved | Non-small-cell lung cancer | [3], [4] | |
| 3 | Cetuximab | Drug Info | Approved | Colorectal cancer | [5], [6] | |
| 4 | Dacomitinib | Drug Info | Approved | Non-small-cell lung cancer | [7] | |
| 5 | Epidermal growth factor | Drug Info | Approved | Vulnerary | [8] | |
| 6 | Erlotinib | Drug Info | Approved | Non-small-cell lung cancer | [6], [9] | |
| 7 | Gefitinib | Drug Info | Approved | Solid tumour/cancer | [10], [11] | |
| 8 | HEGF | Drug Info | Approved | Diabetic foot ulcer | [12] | |
| 9 | Lapatinib | Drug Info | Approved | Breast cancer | [13], [14] | |
| 10 | Merimepodib | Drug Info | Approved | Breast cancer | [15] | |
| 11 | Necitumumab | Drug Info | Approved | Colorectal cancer | [16], [17] | |
| 12 | NERATINIB MALEATE | Drug Info | Approved | HER2/NEU overexpressing breast cancer | [18] | |
| 13 | Nitroglycerin | Drug Info | Approved | Diabetic foot ulcer | [12] | |
| 14 | Osimertinib | Drug Info | Approved | Non-small-cell lung cancer | [19] | |
| 15 | Panitumumab | Drug Info | Approved | Colorectal cancer | [20], [21] | |
| 16 | SKI-758 | Drug Info | Approved | Ischemia | [8], [22] | |
| 17 | Sorafenib | Drug Info | Approved | Renal cell carcinoma | [23], [24] | |
| 18 | Vandetanib | Drug Info | Approved | Solid tumour/cancer | [25], [26] | |
| 19 | Icotinib hydrochloride | Drug Info | Registered | Non-small-cell lung cancer | [27], [28], [29] | |
| Clinical Trial Drug(s) | [+] 66 Clinical Trial Drugs | + | ||||
| 1 | A140 | Drug Info | Phase 3 | Head and neck cancer | [30] | |
| 2 | Almonertinib | Drug Info | Phase 3 | Non-small-cell lung cancer | [31] | |
| 3 | ASP1929 | Drug Info | Phase 3 | Head and neck cancer | [32] | |
| 4 | Bevacizumab + Erlotinib | Drug Info | Phase 3 | Metastatic colorectal cancer | [33], [34] | |
| 5 | CO-1686 | Drug Info | Phase 3 | Lung cancer | [35] | |
| 6 | DE-766 | Drug Info | Phase 3 | Non-small-cell lung cancer | [36], [37] | |
| 7 | EGF816 | Drug Info | Phase 3 | Non-small-cell lung cancer | [38] | |
| 8 | HKI-272 | Drug Info | Phase 3 | Breast cancer | [39], [38] | |
| 9 | Rindopepimut | Drug Info | Phase 3 | Glioblastoma multiforme | [40] | |
| 10 | SYM-004 | Drug Info | Phase 3 | Metastatic colorectal cancer | [38] | |
| 11 | Zalutumumab | Drug Info | Phase 3 | Head and neck cancer | [41] | |
| 12 | Indium-111 | Drug Info | Phase 2/3 | Solid tumour/cancer | [42] | |
| 13 | Varlitinib | Drug Info | Phase 2/3 | Metastatic biliary tract neoplasms | [38] | |
| 14 | ABT-414 | Drug Info | Phase 2 | Glioblastoma multiforme | [43], [44] | |
| 15 | ASP8273 | Drug Info | Phase 2 | Non-small-cell lung cancer | [45] | |
| 16 | BMS-599626 | Drug Info | Phase 2 | Solid tumour/cancer | [46], [47] | |
| 17 | BMS-690514 | Drug Info | Phase 2 | Chronic pain | [48] | |
| 18 | CetuGEX | Drug Info | Phase 2 | Solid tumour/cancer | [49] | |
| 19 | CI-1033 | Drug Info | Phase 2 | Lymphoma | [50], [51] | |
| 20 | Depatuxizumab | Drug Info | Phase 2 | Glioblastoma of brain | [52] | |
| 21 | HER1-VSSP vaccine | Drug Info | Phase 2 | Solid tumour/cancer | [53], [54] | |
| 22 | HM-78136B | Drug Info | Phase 2 | Solid tumour/cancer | [55], [56] | |
| 23 | Matuzumab | Drug Info | Phase 2 | Gastric adenocarcinoma | [57] | |
| 24 | MEHD-7945A | Drug Info | Phase 2 | Colorectal cancer | [58], [59] | |
| 25 | Pazopanib + Tyverb/Tykerb | Drug Info | Phase 2 | Inflammatory breast cancer | [60] | |
| 26 | Pelitinib | Drug Info | Phase 2 | Lymphoma | [61], [62] | |
| 27 | RM-1929 | Drug Info | Phase 2 | Head and neck cancer | [38], [63] | |
| 28 | Tarloxotinib | Drug Info | Phase 2 | Non-small-cell lung cancer | [64] | |
| 29 | TT-100 | Drug Info | Phase 2 | Non-small-cell lung cancer | [65] | |
| 30 | VATALANIB | Drug Info | Phase 2 | Solid tumour/cancer | [66], [67] | |
| 31 | AFM24 | Drug Info | Phase 1/2 | Solid tumour/cancer | [68] | |
| 32 | BDTX-189 | Drug Info | Phase 1/2 | Solid tumour/cancer | [69] | |
| 33 | CART-EGFR | Drug Info | Phase 1/2 | Solid tumour/cancer | [70] | |
| 34 | EGFR CART | Drug Info | Phase 1/2 | Colorectal cancer | [71] | |
| 35 | EMB-01 | Drug Info | Phase 1/2 | Neoplasm | [72] | |
| 36 | EMD 55900 | Drug Info | Phase 1/2 | Glioma | [73] | |
| 37 | SN-32793 | Drug Info | Phase 1/2 | Non-small-cell lung cancer | [74] | |
| 38 | Sym013 | Drug Info | Phase 1/2 | Epithelial ovarian cancer | [38] | |
| 39 | Sym015 | Drug Info | Phase 1/2 | Solid tumour/cancer | [63], [38] | |
| 40 | TAK-186 | Drug Info | Phase 1/2 | Aggressive cancer | [75] | |
| 41 | ZN-e4 | Drug Info | Phase 1/2 | Non-small-cell lung cancer | [76] | |
| 42 | S-222611 | Drug Info | Phase 1b | Malignant solid tumour | [77] | |
| 43 | AMG 595 | Drug Info | Phase 1 | Glioblastoma multiforme | [78] | |
| 44 | Anti-EGFR CAR T | Drug Info | Phase 1 | Glioma | [79] | |
| 45 | Anti-HER3/EGFR DAF | Drug Info | Phase 1 | Metastatic epithelial tumour | [80] | |
| 46 | AP32788 | Drug Info | Phase 1 | Non-small-cell lung cancer | [38] | |
| 47 | AST-1306 | Drug Info | Phase 1 | Solid tumour/cancer | [81] | |
| 48 | AZD9592 | Drug Info | Phase 1 | Aggressive cancer | [82] | |
| 49 | BCA101 | Drug Info | Phase 1 | Solid tumour/cancer | [83] | |
| 50 | BIBX-1382 | Drug Info | Phase 1 | Chronic lymphocytic leukaemia | [84], [85] | |
| 51 | Cipatinib | Drug Info | Phase 1 | Solid tumour/cancer | [86] | |
| 52 | CLN-081 | Drug Info | Phase 1 | Non-small-cell lung cancer | [87] | |
| 53 | CUDC-101 | Drug Info | Phase 1 | Solid tumour/cancer | [88] | |
| 54 | CX-904 | Drug Info | Phase 1 | Colorectal cancer | [89] | |
| 55 | D2C7 | Drug Info | Phase 1 | Glioblastoma of brain | [90] | |
| 56 | EGFR806-specific CAR T cell | Drug Info | Phase 1 | Atypical teratoid/rhabdoid tumour | [91] | |
| 57 | HER-2/HER-1 vaccine | Drug Info | Phase 1 | Solid tumour/cancer | [92] | |
| 58 | IMGN289 | Drug Info | Phase 1 | Solid tumour/cancer | [93] | |
| 59 | JNJ-26483327 | Drug Info | Phase 1 | Solid tumour/cancer | [94] | |
| 60 | LY3164530 | Drug Info | Phase 1 | Advanced cancer | [95] | |
| 61 | MCLA-158 | Drug Info | Phase 1 | Solid tumour/cancer | [96] | |
| 62 | MM-151 | Drug Info | Phase 1 | Solid tumour/cancer | [97] | |
| 63 | MR1-1 | Drug Info | Phase 1 | Brain cancer | [98] | |
| 64 | PF-05230907 | Drug Info | Phase 1 | Nontraumatic intracerebral hemorrhage | [99] | |
| 65 | SI-B001 | Drug Info | Phase 1 | Metastatic epithelial tumour | [100] | |
| 66 | SYN004 | Drug Info | Phase 1 | Solid tumour/cancer | [63], [38] | |
| Discontinued Drug(s) | [+] 8 Discontinued Drugs | + | ||||
| 1 | PKI166 | Drug Info | Discontinued in Phase 2 | Esophageal cancer | [101], [102] | |
| 2 | RG7160 | Drug Info | Discontinued in Phase 2 | Metastatic colorectal cancer | [103] | |
| 3 | AZD4769 | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [104] | |
| 4 | PD-153035 | Drug Info | Discontinued in Phase 1 | Psoriasis vulgaris | [105] | |
| 5 | TAK165 | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [106], [107] | |
| 6 | AZD-9935 | Drug Info | Terminated | Solid tumour/cancer | [111] | |
| 7 | CGP-52411 | Drug Info | Terminated | Solid tumour/cancer | [112] | |
| 8 | Heparin-EGF-like factor | Drug Info | Terminated | Gastrointestinal disease | [113], [114] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | 111In-hEGF | Drug Info | Preclinical | Breast cancer | [108], [109] | |
| 2 | EGFR/IGFR tandem adnectin | Drug Info | Preclinical | Solid tumour/cancer | [110] | |
| Mode of Action | [+] 6 Modes of Action | + | ||||
| Inhibitor | [+] 128 Inhibitor drugs | + | ||||
| 1 | Amivantamab | Drug Info | [115] | |||
| 2 | BIBW 2992 | Drug Info | [116] | |||
| 3 | Erlotinib | Drug Info | [120] | |||
| 4 | Gefitinib | Drug Info | [121], [122] | |||
| 5 | Lapatinib | Drug Info | [123], [124] | |||
| 6 | Merimepodib | Drug Info | [125] | |||
| 7 | Osimertinib | Drug Info | [127] | |||
| 8 | SKI-758 | Drug Info | [129] | |||
| 9 | Sorafenib | Drug Info | [130] | |||
| 10 | Vandetanib | Drug Info | [131], [132] | |||
| 11 | Almonertinib | Drug Info | [134] | |||
| 12 | ASP1929 | Drug Info | [135] | |||
| 13 | Bevacizumab + Erlotinib | Drug Info | [136] | |||
| 14 | EGF816 | Drug Info | [138] | |||
| 15 | HKI-272 | Drug Info | [139] | |||
| 16 | ASP8273 | Drug Info | [145] | |||
| 17 | BMS-599626 | Drug Info | [146], [131] | |||
| 18 | BMS-690514 | Drug Info | [147] | |||
| 19 | CI-1033 | Drug Info | [131] | |||
| 20 | HM-78136B | Drug Info | [38], [148] | |||
| 21 | Pazopanib + Tyverb/Tykerb | Drug Info | [125] | |||
| 22 | Tarloxotinib | Drug Info | [152] | |||
| 23 | TT-100 | Drug Info | [153] | |||
| 24 | VATALANIB | Drug Info | [139] | |||
| 25 | BDTX-189 | Drug Info | [155] | |||
| 26 | EMB-01 | Drug Info | [156] | |||
| 27 | SN-32793 | Drug Info | [119] | |||
| 28 | TAK-186 | Drug Info | [157] | |||
| 29 | ZN-e4 | Drug Info | [158] | |||
| 30 | S-222611 | Drug Info | [77] | |||
| 31 | AP32788 | Drug Info | [38] | |||
| 32 | BCA101 | Drug Info | [83] | |||
| 33 | CLN-081 | Drug Info | [166] | |||
| 34 | IMGN289 | Drug Info | [169] | |||
| 35 | JNJ-26483327 | Drug Info | [170] | |||
| 36 | MCLA-158 | Drug Info | [171] | |||
| 37 | PF-05230907 | Drug Info | [173] | |||
| 38 | SYN004 | Drug Info | [63] | |||
| 39 | Pyrrolo[2,3-d]pyrimidine derivative 24 | Drug Info | [175] | |||
| 40 | AZD4769 | Drug Info | [132] | |||
| 41 | TAK165 | Drug Info | [131] | |||
| 42 | EGFR/IGFR tandem adnectin | Drug Info | [180] | |||
| 43 | CGP-52411 | Drug Info | [182] | |||
| 44 | CGP-53353 | Drug Info | [183] | |||
| 45 | (3-Bromo-phenyl)-(5-nitro-quinazolin-4-yl)-amine | Drug Info | [184] | |||
| 46 | (3-Bromo-phenyl)-quinazolin-4-yl-amine | Drug Info | [185] | |||
| 47 | (E)-5-(4-Hydroxybenzylidene)-1-phenethylhydantoin | Drug Info | [186] | |||
| 48 | (S)-benzyl 4-chloro-3-oxobutan-2-ylcarbamate | Drug Info | [187] | |||
| 49 | 10-hydroxy-18-methoxybetaenone | Drug Info | [188] | |||
| 50 | 2-(4,5-Dihydroxy-indan-1-ylidene)-malononitrile | Drug Info | [189] | |||
| 51 | 2-(5,6-Dihydroxy-indan-1-ylidene)-malononitrile | Drug Info | [189] | |||
| 52 | 2-benzoyl-3-(3,4-dihydroxyphenyl)acrylonitrile | Drug Info | [187] | |||
| 53 | 2-cyano-3-(3,4-dihydroxyphenyl)acrylamide | Drug Info | [187] | |||
| 54 | 2-methoxy-4-(2-nitrovinyl)phenol | Drug Info | [187] | |||
| 55 | 3,4-di-(4-methoxyphenyl)-1H-pyrrole-2,5-dione | Drug Info | [190] | |||
| 56 | 3,4-diphenyl-1H-pyrrole-2,5-dione | Drug Info | [190] | |||
| 57 | 3-(3-Chloro-phenyl)-5,7-dihydroxy-chromen-4-one | Drug Info | [191] | |||
| 58 | 3-(4-methoxyphenyl)-4-phenyl-1H-pyrrole-2,5-dione | Drug Info | [190] | |||
| 59 | 3-(indole-3-yl)-4-phenyl-1H-pyrrole-2,5-dione | Drug Info | [190] | |||
| 60 | 3-Pyridin-4-yl-quinoline-6,7-diol | Drug Info | [192] | |||
| 61 | 4-(2-nitroprop-1-enyl)benzene-1,2-diol | Drug Info | [187] | |||
| 62 | 4-(2-nitrovinyl)benzene-1,2-diol | Drug Info | [187] | |||
| 63 | 4-(2-nitrovinyl)phenol | Drug Info | [187] | |||
| 64 | 4-(3-Bromo-phenoxy)-6,7-dimethoxy-quinazoline | Drug Info | [193] | |||
| 65 | 4-(3-Bromo-phenoxy)-6,7-dimethoxy-quinoline | Drug Info | [193] | |||
| 66 | 4-(3-Bromo-phenylamino)-quinazoline-6,7-diol | Drug Info | [185] | |||
| 67 | 4-(4-(2-nitrovinyl)phenoxysulfonyl)benzoic acid | Drug Info | [187] | |||
| 68 | 4-(5-Bromoindole-3-yl)-6,7-dimethoxyquinazoline | Drug Info | [194] | |||
| 69 | 4-acrylamido-N-(3-bromophenyl)-2-hydroxybenzamide | Drug Info | [195] | |||
| 70 | 4-biphenyl-2-ylethynyl-6,7-dimethoxy-quinazoline | Drug Info | [196] | |||
| 71 | 4557W | Drug Info | [197] | |||
| 72 | 5,6-Bis-p-tolylamino-isoindole-1,3-dione | Drug Info | [183] | |||
| 73 | 5-acrylamido-N-(3-bromophenyl)-2-hydroxybenzamide | Drug Info | [195] | |||
| 74 | 6,7-diethoxy-4-(4-phenylbut-1-enyl)quinazoline | Drug Info | [196] | |||
| 75 | 6,7-diethoxy-4-(5-phenylpent-1-enyl)quinazoline | Drug Info | [196] | |||
| 76 | 6,7-diethoxy-4-styrylquinazoline | Drug Info | [196] | |||
| 77 | 6,7-dimethoxy-4-(2-phenylethynyl)quinazoline | Drug Info | [196] | |||
| 78 | 6,7-dimethoxy-4-(3-phenoxyprop-1-ynyl)quinazoline | Drug Info | [196] | |||
| 79 | 6,7-dimethoxy-4-(4-phenylbut-1-ynyl)quinazoline | Drug Info | [196] | |||
| 80 | 6,7-dimethoxy-N-m-tolylquinazolin-4-amine | Drug Info | [198] | |||
| 81 | 6-chloro-N-(3-chlorophenyl)quinazolin-4-amine | Drug Info | [199] | |||
| 82 | AG 112 | Drug Info | [200] | |||
| 83 | AG 9 | Drug Info | [200] | |||
| 84 | AG-213 | Drug Info | [194] | |||
| 85 | AG-538 | Drug Info | [187] | |||
| 86 | Benzo[g]quinazolin-4-yl-(3-bromo-phenyl)-amine | Drug Info | [185] | |||
| 87 | Benzyl-quinazolin-4-yl-amine | Drug Info | [184] | |||
| 88 | BPIQ-I | Drug Info | [185] | |||
| 89 | CL-387785 | Drug Info | [198] | |||
| 90 | Cochliobolic acid | Drug Info | [201] | |||
| 91 | EGFR inhibitor | Drug Info | [202] | |||
| 92 | Epitinib | Drug Info | [119] | |||
| 93 | HDS-029 | Drug Info | [203] | |||
| 94 | HKI-9924129 | Drug Info | [204] | |||
| 95 | HM-61713B | Drug Info | [119] | |||
| 96 | HTS-00213 | Drug Info | [198] | |||
| 97 | HTS-02876 | Drug Info | [198] | |||
| 98 | HTS-05058 | Drug Info | [202] | |||
| 99 | LAVENDUSTIN A | Drug Info | [205] | |||
| 100 | MDP-01 | Drug Info | [119] | |||
| 101 | MT-062 | Drug Info | [119] | |||
| 102 | N*4*-(3-Bromo-phenyl)-quinazoline-4,6,7-triamine | Drug Info | [185] | |||
| 103 | N*4*-(3-Bromo-phenyl)-quinazoline-4,6-diamine | Drug Info | [207] | |||
| 104 | N*4*-(3-Bromo-phenyl)-quinazoline-4,7-diamine | Drug Info | [184] | |||
| 105 | N*4*-Benzyl-pyrido[4,3-d]pyrimidine-4,7-diamine | Drug Info | [208] | |||
| 106 | N-(4-(phenylamino)quinazolin-6-yl)acrylamide | Drug Info | [209] | |||
| 107 | N-(4-m-Tolylamino-quinazolin-6-yl)-acrylamide | Drug Info | [210] | |||
| 108 | N-(4-m-Tolylamino-quinazolin-7-yl)-acrylamide | Drug Info | [210] | |||
| 109 | N4-(3-chlorophenyl)quinazoline-4,6-diamine | Drug Info | [211] | |||
| 110 | N4-(3-methylphenyl)-4,6-quinazolinediamine | Drug Info | [212] | |||
| 111 | NRC-2694 | Drug Info | [119] | |||
| 112 | ON-128 | Drug Info | [119] | |||
| 113 | OSI-75 | Drug Info | [213] | |||
| 114 | PD-0166326 | Drug Info | [204] | |||
| 115 | PD-0173956 | Drug Info | [204] | |||
| 116 | PD-158780 | Drug Info | [208] | |||
| 117 | PD-168393 | Drug Info | [209] | |||
| 118 | PD182905 | Drug Info | [214] | |||
| 119 | PMID24915291C38 | Drug Info | [216] | |||
| 120 | PMID8568816C56 | Drug Info | [217] | |||
| 121 | PP121 | Drug Info | [218] | |||
| 122 | RG-50810 | Drug Info | [187] | |||
| 123 | RM-6427 | Drug Info | [119] | |||
| 124 | Ro-4396686 | Drug Info | [219] | |||
| 125 | Theliatinib | Drug Info | [119] | |||
| 126 | Tyrphostin ag-1478 | Drug Info | [191] | |||
| 127 | WHI-P154 | Drug Info | [220] | |||
| 128 | WZ-3146 | Drug Info | [119] | |||
| Antagonist | [+] 13 Antagonist drugs | + | ||||
| 1 | Dacomitinib | Drug Info | [119] | |||
| 2 | NERATINIB MALEATE | Drug Info | [18] | |||
| 3 | Icotinib hydrochloride | Drug Info | [133] | |||
| 4 | Depatuxizumab | Drug Info | [63] | |||
| 5 | RM-1929 | Drug Info | [38] | |||
| 6 | Sym013 | Drug Info | [63], [38] | |||
| 7 | Sym015 | Drug Info | [63] | |||
| 8 | BIBX-1382 | Drug Info | [165] | |||
| 9 | MM-151 | Drug Info | [63] | |||
| 10 | AZD-9935 | Drug Info | [181] | |||
| 11 | EDP-13 | Drug Info | [119] | |||
| 12 | IPS-01003 | Drug Info | [119] | |||
| 13 | RX-1792 | Drug Info | [119] | |||
| Modulator | [+] 27 Modulator drugs | + | ||||
| 1 | Epidermal growth factor | Drug Info | [119] | |||
| 2 | Necitumumab | Drug Info | [126] | |||
| 3 | CO-1686 | Drug Info | [137] | |||
| 4 | DE-766 | Drug Info | [119] | |||
| 5 | Rindopepimut | Drug Info | [126] | |||
| 6 | Varlitinib | Drug Info | [142] | |||
| 7 | MEHD-7945A | Drug Info | [142], [150] | |||
| 8 | Pelitinib | Drug Info | [151] | |||
| 9 | AMG 595 | Drug Info | [159] | |||
| 10 | Anti-HER3/EGFR DAF | Drug Info | [160] | |||
| 11 | AST-1306 | Drug Info | [161], [162], [163] | |||
| 12 | Cipatinib | Drug Info | [142] | |||
| 13 | CUDC-101 | Drug Info | [88] | |||
| 14 | LY3164530 | Drug Info | [150] | |||
| 15 | MR1-1 | Drug Info | [172] | |||
| 16 | PKI166 | Drug Info | [176] | |||
| 17 | RG7160 | Drug Info | [177] | |||
| 18 | PD-153035 | Drug Info | [178] | |||
| 19 | 111In-hEGF | Drug Info | [179] | |||
| 20 | Heparin-EGF-like factor | Drug Info | [113], [114] | |||
| 21 | AGT-2000 | Drug Info | [119] | |||
| 22 | AL-6802 | Drug Info | [119] | |||
| 23 | LA22-radioimmunoconjugates | Drug Info | [119] | |||
| 24 | MG-111 | Drug Info | [206] | |||
| 25 | PF 5208766 | Drug Info | [215] | |||
| 26 | SYM-011 | Drug Info | [119] | |||
| 27 | TGF alpha | Drug Info | [119] | |||
| Activator | [+] 2 Activator drugs | + | ||||
| 1 | HEGF | Drug Info | [12] | |||
| 2 | Nitroglycerin | Drug Info | [12] | |||
| Suppressor | [+] 1 Suppressor drugs | + | ||||
| 1 | Panitumumab | Drug Info | [128] | |||
| CAR-T-Cell-Therapy | [+] 4 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | CART-EGFR | Drug Info | [70] | |||
| 2 | EGFR CART | Drug Info | [71] | |||
| 3 | Anti-EGFR CAR T | Drug Info | [79] | |||
| 4 | EGFR806-specific CAR T cell | Drug Info | [91] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Lapatinib | Ligand Info | |||||
| Structure Description | EGFR kinase domain complexed with a quinazoline inhibitor- GW572016 | PDB:1XKK | ||||
| Method | X-ray diffraction | Resolution | 2.40 Å | Mutation | No | [222] |
| PDB Sequence |
ALLRILKETE
711 FKKIKVLGSG721 AFGTVYKGLW731 IPVKIPVAIK745 ELREKANKEI759 LDEAYVMASV 769 DNPHVCRLLG779 ICLTSTVQLI789 TQLMPFGCLL799 DYVREHKDNI809 GSQYLLNWCV 819 QIAKGMNYLE829 DRRLVHRDLA839 ARNVLVKTPQ849 HVKITDFGLA859 KLLGAEEKVP 877 IKWMALESIL887 HRIYTHQSDV897 WSYGVTVWEL907 MTFGSKPYDG917 IPASEISSIL 927 EKGERLPQPP937 ICTIDVYMIM947 VKCWMIDADS957 RPKFRELIIE967 FSKMARDPQR 977 YLVIQGDERM987 SNFYRALMDE1004 VVDADEYLI
|
|||||
|
|
LEU718
3.870
GLY719
4.124
VAL726
3.854
ALA743
3.334
ILE744
4.349
LYS745
3.414
MET766
3.317
CYS775
3.424
ARG776
3.123
LEU777
3.371
LEU788
3.100
ILE789
4.160
THR790
3.138
GLN791
3.195
|
|||||
| Ligand Name: Brigatinib | Ligand Info | |||||
| Structure Description | Studies Towards a Reversible EGFR C797S Triple Mutant Inhibitor Series | PDB:7AEM | ||||
| Method | X-ray diffraction | Resolution | 2.65 Å | Mutation | No | [223] |
| PDB Sequence |
SGEAPNQALL
704 RILKETEFKK714 IKVLGSGAFG724 TVYKGLWIPE734 GEKVKIPVAI744 KELATSPKAN 756 KEILDEAYVM766 ASVDNPHVCR776 LLGICLTSTV786 QLITQLMPFG796 CLLDYVREHK 806 DNIGSQYLLN816 WCVQIAKGMN826 YLEDRRLVHR836 DLAARNVLVK846 TPQHVKITDF 856 GLAKLLGAEE866 KEYHAEGGKV876 PIKWMALESI886 LHRIYTHQSD896 VWSYGVTVWE 906 LMTFGSKPYD916 GIPASEISSI926 LEKGERLPQP936 PICTIDVYMI946 MVKCWMIDAD 956 SRPKFRELII966 EFSKMARDPQ976 RYLVIQGDER986 MHLPLMDEED1006 MDDVVDADEY 1016 LI
|
|||||
|
|
LEU718
3.663
GLY719
3.649
PHE723
3.562
VAL726
3.766
ALA743
3.266
LYS745
4.226
THR790
3.893
GLN791
3.498
LEU792
3.620
MET793
2.921
PRO794
3.438
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| ErbB signaling pathway | hsa04012 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Rap1 signaling pathway | hsa04015 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| FoxO signaling pathway | hsa04068 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Endocytosis | hsa04144 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Focal adhesion | hsa04510 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Adherens junction | hsa04520 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Gap junction | hsa04540 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Regulation of actin cytoskeleton | hsa04810 | Affiliated Target |

|
| Class: Cellular Processes => Cell motility | Pathway Hierarchy | ||
| GnRH signaling pathway | hsa04912 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
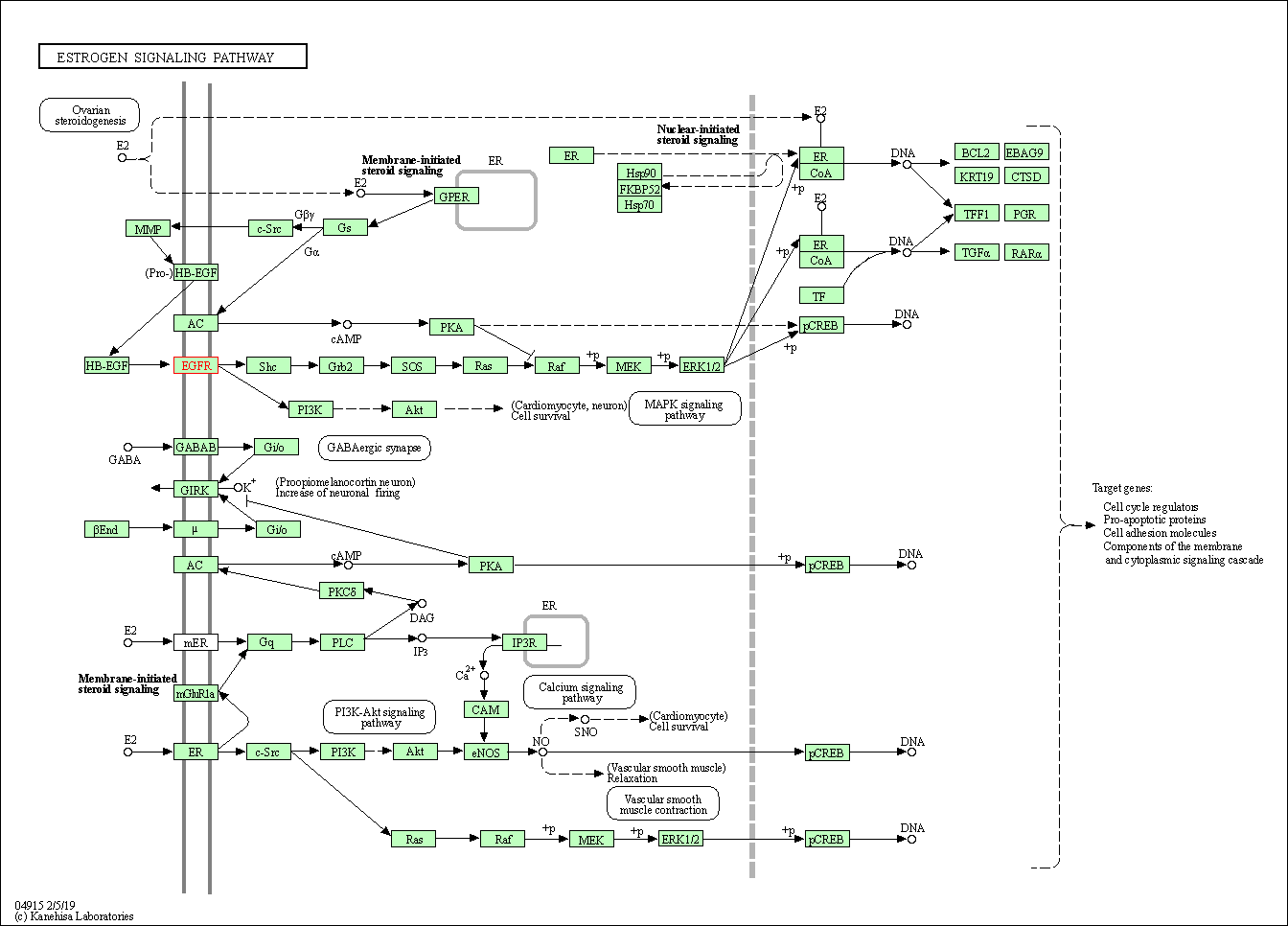
| Estrogen signaling pathway | hsa04915 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
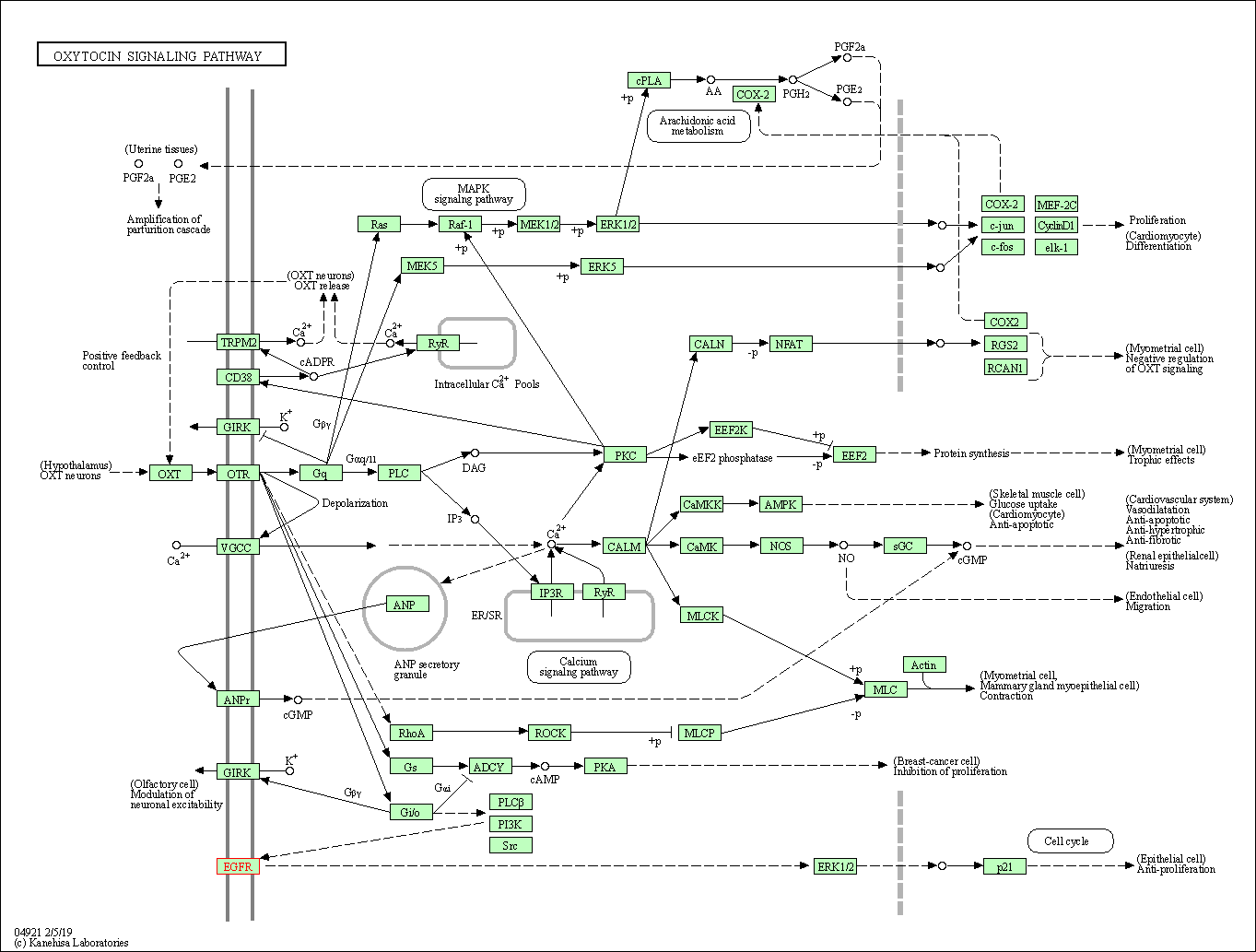
| Oxytocin signaling pathway | hsa04921 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
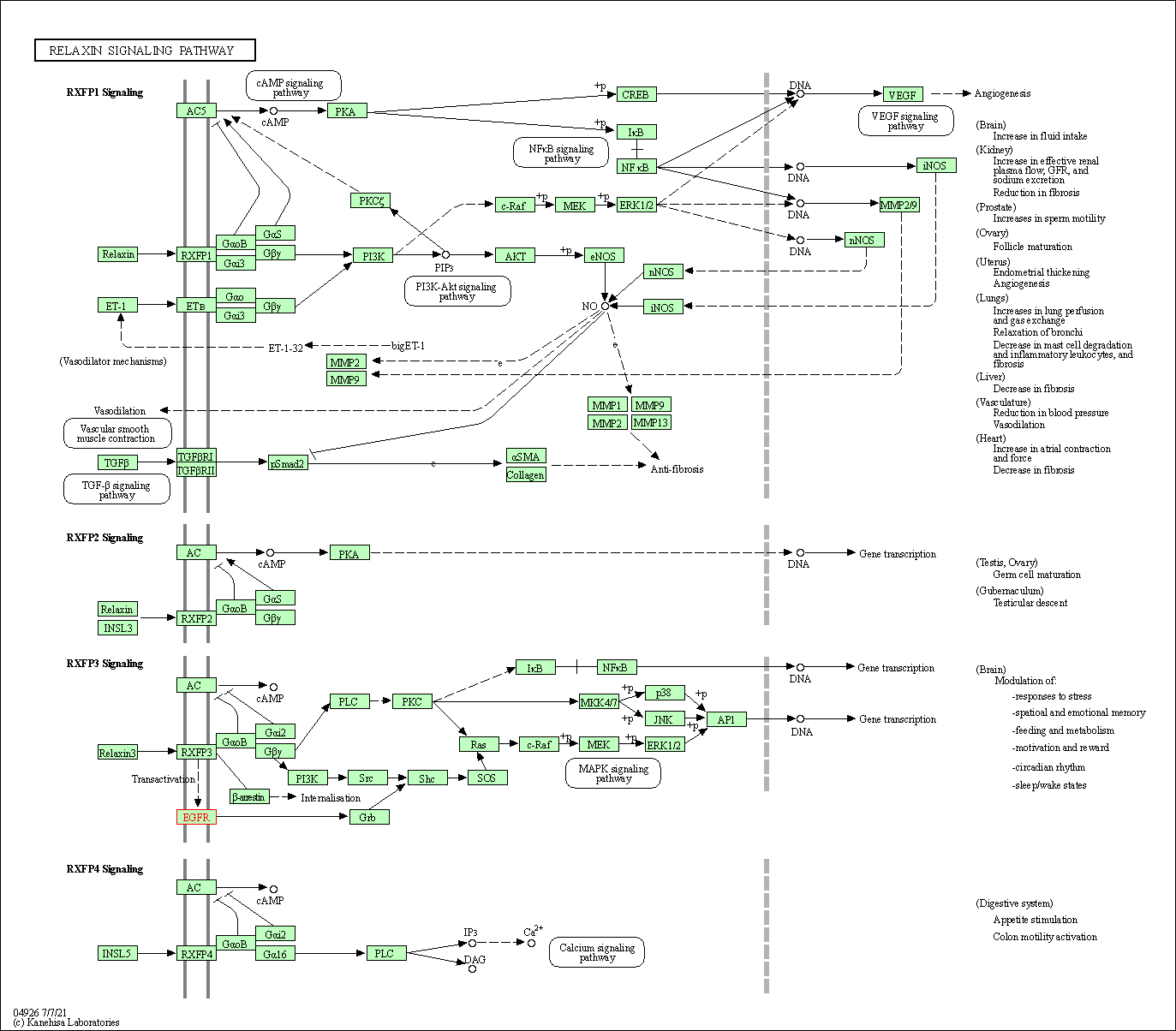
| Relaxin signaling pathway | hsa04926 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
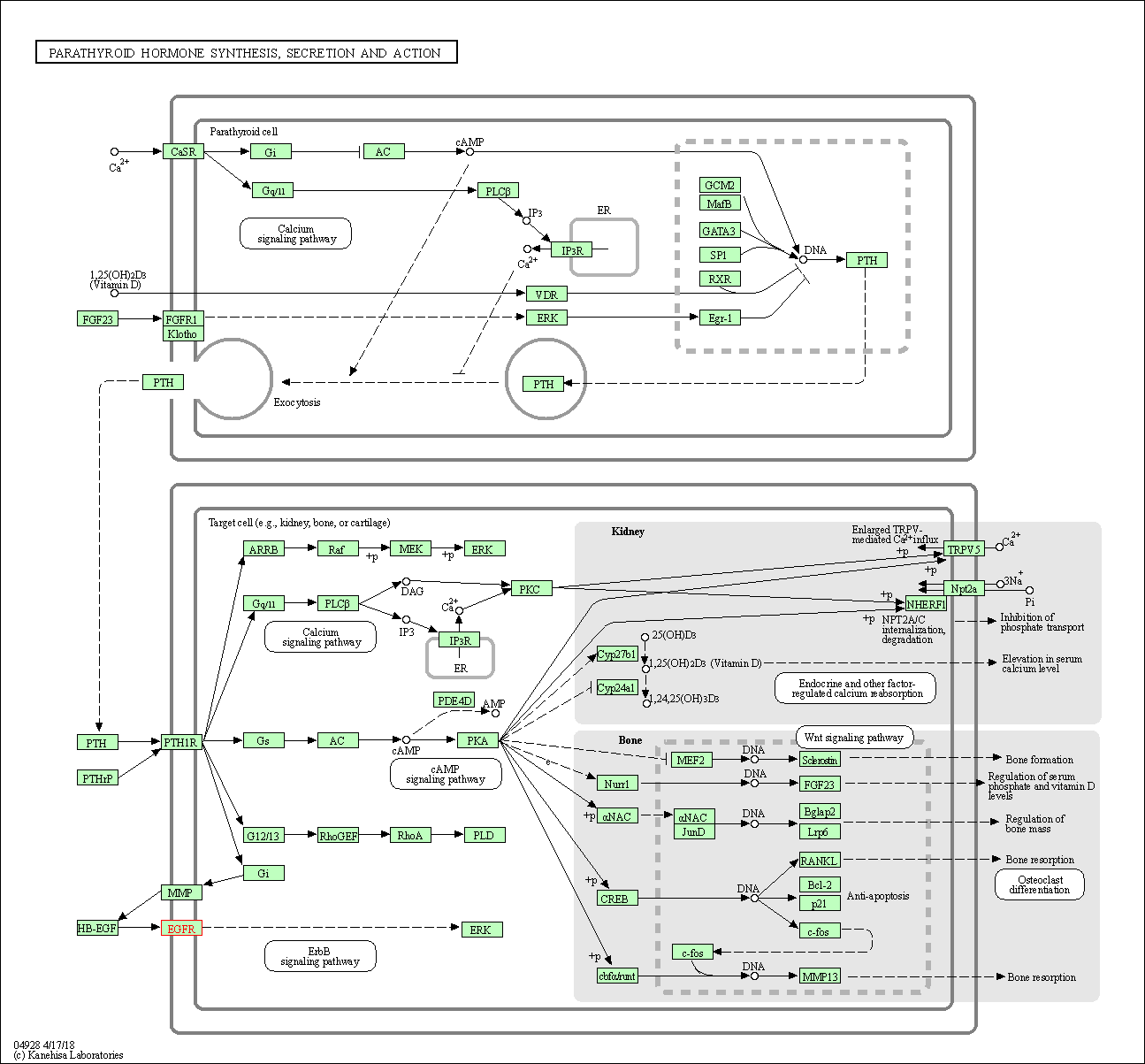
| Parathyroid hormone synthesis, secretion and action | hsa04928 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 137 | Degree centrality | 1.47E-02 | Betweenness centrality | 2.54E-02 |
|---|---|---|---|---|---|
| Closeness centrality | 2.82E-01 | Radiality | 1.48E+01 | Clustering coefficient | 6.91E-02 |
| Neighborhood connectivity | 3.41E+01 | Topological coefficient | 2.04E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Drug Resistance Mutation (DRM) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. 2002 May;1(7):507-14. | |||||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 761210. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5667). | |||||
| REF 4 | BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008 Dec;9(12):1336-46. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6882). | |||||
| REF 6 | 2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4. | |||||
| REF 7 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4920). | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4941). | |||||
| REF 11 | Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2006 May;11(2):275-91. | |||||
| REF 12 | Emerging drugs for diabetic foot ulcers. Expert Opin Emerg Drugs. 2006 Nov;11(4):709-24. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5692). | |||||
| REF 14 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 022059. | |||||
| REF 15 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 022059. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8090). | |||||
| REF 17 | ClinicalTrials.gov (NCT00981058) First-line Treatment of Participants With Stage IV Squamous Non-Small Cell Lung Cancer With Necitumumab and Gemcitabine-Cisplatin. U.S. National Institutes of Health. | |||||
| REF 18 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 19 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6883). | |||||
| REF 21 | 2006 drug approvals: finding the niche. Nat Rev Drug Discov. 2007 Feb;6(2):99-101. | |||||
| REF 22 | Nat Rev Drug Discov. 2013 Feb;12(2):87-90. | |||||
| REF 23 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5711). | |||||
| REF 24 | Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597-612. | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5717). | |||||
| REF 26 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||||
| REF 27 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7641). | |||||
| REF 28 | Clinical pipeline report, company report or official report of Beta Pharma. | |||||
| REF 29 | ClinicalTrials.gov (NCT02404675) High Dose Icotinib in Advanced Non-small Cell Lung Cancer Patients With EGFR 21 Exon Positive. U.S. National Institutes of Health. | |||||
| REF 30 | Clinical pipeline report, company report or official report of Klus Pharma | |||||
| REF 31 | ClinicalTrials.gov (NCT04870190) Almonertinib Versus Osimertinib in the Patients With EGFR Mutations in Advanced NSCLC With Brain Metastases (ATTACK). U.S. National Institutes of Health. | |||||
| REF 32 | ClinicalTrials.gov (NCT03769506) ASP-1929 Photoimmunotherapy (PIT) Study in Recurrent Head/Neck Cancer for Patients Who Have Failed at Least Two Lines of Therapy. U.S. National Institutes of Health. | |||||
| REF 33 | ClinicalTrials.gov (NCT00130728) A Study to Evaluate the Efficacy of Bevacizumab in Combination With Tarceva for Advanced Non-Small Cell Lung Cancer | |||||
| REF 34 | ClinicalTrials.gov (NCT00598156) Chemotherapy and Avastin Followed by Maintenance Treatment With Avastin +/- Tarceva | |||||
| REF 35 | ClinicalTrials.gov (NCT02322281) TIGER-3: Open Label, Multicenter Study of Rociletinib (CO-1686) Mono Therapy Versus Single-agent Cytotoxic Chemotherapy in Patients With Mutant EGFR NSCLC Who Have Failed at Least One Previous EGFR-Directed TKI and Platinum-doublet Chemotherapy | |||||
| REF 36 | Clinical pipeline report, company report or official report of Daiichi Sankyo. | |||||
| REF 37 | Clinical pipeline report, company report or official report of Daiichi Sankyo. | |||||
| REF 38 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 39 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021154) | |||||
| REF 40 | ClinicalTrials.gov (NCT01480479) Phase III Study of Rindopepimut/GM-CSF in Patients With Newly Diagnosed Glioblastoma. U.S. National Institutes of Health. | |||||
| REF 41 | ClinicalTrials.gov (NCT00382031) Zalutumumab in Patients With Non-curable Head and Neck Cancer. U.S. National Institutes of Health. | |||||
| REF 42 | ClinicalTrials.gov (NCT00442533) Safety and Efficacy Study of In-111 Pentetreotide to Treat Neuroendocrine Tumors. U.S. National Institutes of Health. | |||||
| REF 43 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7970). | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035129) | |||||
| REF 45 | ClinicalTrials.gov (NCT02500927) A Study of ASP8273 in Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor-Naive Patients With Non-Small Cell Lung Cancer Harboring EGFR Mutations. | |||||
| REF 46 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7647). | |||||
| REF 47 | ClinicalTrials.gov (NCT01614522) A Clinical Trial Evaluating the Effect of ASLAN001 in Patients With Recurrent/Metastatic Gastric Cancer Whose Tumors Are Either HER-2 Amplified or Co-expressing HER-1and HER-2. U.S. National Institutes of Health. | |||||
| REF 48 | A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007 Jul 1;67(13):6253-62. | |||||
| REF 49 | Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol Ther. 2011 May 1;11(9):777-92. | |||||
| REF 50 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5675). | |||||
| REF 51 | Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4-(phenylamino)quinazoline- and 4-(phenylamino)pyrido[3,2-d]pyrimidine-6-acrylamides bearing additional solubilizing functions. J Med Chem. 2000 Apr 6;43(7):1380-97. | |||||
| REF 52 | ClinicalTrials.gov (NCT02343406) Adult Study: ABT-414 Alone or ABT-414 Plus Temozolomide vs. Lomustine or Temozolomide for Recurrent Glioblastoma Pediatric Study: Evaluation of ABT-414 in Children With High Grade Gliomas (INTELLANCE-2). U.S. National Institutes of Health. | |||||
| REF 53 | Active antimetastatic immunotherapy in Lewis lung carcinoma with self EGFR extracellular domain protein in VSSP adjuvant. Int J Cancer. 2006 Nov 1;119(9):2190-9. | |||||
| REF 54 | HER1-ECD vaccination dispenses with emulsification to elicit HER1-specific anti-proliferative effects. Hum Vaccin. 2009 Mar;5(3):158-65. | |||||
| REF 55 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7903). | |||||
| REF 56 | ClinicalTrials.gov (NCT02216916) Phase II Trial of HM781-36B in Patients With Metastatic/Recurrent Head and Neck Squamous Cell Carcinoma (HNSCC) After Failure of or Unfit for Platinum-containing Therapy. U.S. National Institutes of Health. | |||||
| REF 57 | ClinicalTrials.gov (NCT00073541) A Study of the Safety and Effects of EMD 72000 in Subjects With Recurrent Ovarian Cancer. U.S. National Institutes of Health. | |||||
| REF 58 | ClinicalTrials.gov (NCT01577173) A Study of MEHD7945A Versus Cetuximab in Patients With Recurrent/Metastatic Squamous Cell Carcinoma of The Head And Neck | |||||
| REF 59 | ClinicalTrials.gov (NCT01652482) A Study of MEHD7945A + FOLFIRI Versus Cetuximab + FOLFIRI in Second Line in Patients With KRAS Wild-Type Metastatic Colorectal Cancer. U.S. National Institutes of Health. | |||||
| REF 60 | A randomized phase II study of lapatinib + pazopanib versus lapatinib in patients with HER2+ inflammatory breast cancer. Breast Cancer Res Treat. 2013 Jan;137(2):471-82. | |||||
| REF 61 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7644). | |||||
| REF 62 | ClinicalTrials.gov (NCT00067548) Study Evaluating EKB-569 in Advanced Non-Small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 63 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 64 | ClinicalTrials.gov (NCT02454842) Study for Treatment of Patients With EGFR Mutant, T790M-negative NSCLC (TH-4000). U.S. National Institutes of Health. | |||||
| REF 65 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021633) | |||||
| REF 66 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5705). | |||||
| REF 67 | ClinicalTrials.gov (NCT00348790) Vatalanib in Treating Patients With Recurrent or Progressive Meningioma. U.S. National Institutes of Health. | |||||
| REF 68 | ClinicalTrials.gov (NCT04259450) Study to Assess AFM24 in Advanced Solid Cancers. U.S. National Institutes of Health. | |||||
| REF 69 | ClinicalTrials.gov (NCT04209465) A Study of BDTX-189, an Orally Available Allosteric ErbB Inhibitor, in Patients With Advanced Solid Tumors. (MasterKey-01). U.S. National Institutes of Health. | |||||
| REF 70 | ClinicalTrials.gov (NCT01869166) Treatment of Chemotherapy Refractory EGFR(Epidermal Growth Factor Receptor) Positive Advanced Solid Tumors (CART-EGFR) | |||||
| REF 71 | ClinicalTrials.gov (NCT03152435) EGFR CART Cells for Patients With Metastatic Colorectal Cancer | |||||
| REF 72 | ClinicalTrials.gov (NCT03797391) A Dose Escalation Study of EMB-01 in Participants With Advanced/Metastatic Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 73 | Multiple infusions of anti-epidermal growth factor receptor (EGFR) monoclonal antibody (EMD 55,900) in patients with recurrent malignant gliomas. Eur J Cancer. 1996 Apr;32A(4):636-40. | |||||
| REF 74 | ClinicalTrials.gov (NCT01631279) A Dose Escalation Trial of PR610 Treating Patients With Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 75 | ClinicalTrials.gov (NCT04844073) A Phase 1/2, First-in-Human, Open-Label, Dose-Escalation Study of TAK-186 (Also Known as MVC-101), An EGFR x CD3 COnditional Bispecific Redirected Activation (COBRA) Protein in Patients With Unresectable Locally Advanced or Metastatic Cancer. U.S.National Institutes of Health. | |||||
| REF 76 | ClinicalTrials.gov (NCT03446417) A Study of ZN-e4 in Subjects With Epidermal Growth Factor Receptor Mutated Non-Small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 77 | Clinical pipeline report, company report or official report of Shionogi (2011). | |||||
| REF 78 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020588) | |||||
| REF 79 | ClinicalTrials.gov (NCT02331693) CAR T Cells in Treating Patients With Malignant Gliomas Overexpressing EGFR | |||||
| REF 80 | Clinical pipeline report, company report or official report of Genentech (2011). | |||||
| REF 81 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800032292) | |||||
| REF 82 | ClinicalTrials.gov (NCT05647122) A Phase I, Multicenter, Open-label, First-in-Human, Dose Escalation and Expansion Study of AZD9592 as Monotherapy and in Combination With Anti-cancer Agents in Patients With Advanced Solid Tumors. U.S.National Institutes of Health. | |||||
| REF 83 | ClinicalTrials.gov (NCT04429542) Study of Safety and Tolerability of BCA101 Alone and in Combination With Pembrolizumab in Patients With EGFR-driven Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 84 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7646). | |||||
| REF 85 | ClinicalTrials.gov (NCT00003980) BIBX 1382 in Treating Patients With Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 86 | ClinicalTrials.gov (NCT01301911) Study of Cipatinib in Patients With HER2 Positive or Uncertain Advanced Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 87 | ClinicalTrials.gov (NCT04036682) A Phase 1/2a Trial of CLN-081 in Patients With Non-Small Cell Lung Cancer. U.S. National Institutes of Health. | |||||
| REF 88 | A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2015 Apr 1;21(7):1566-73. | |||||
| REF 89 | ClinicalTrials.gov (NCT05387265) A Phase 1/1b, Open-label, Dose-finding, First-in-human Study to Evaluate the Safety and Antitumor Activity of CX-904, an EGFR-targeted T-cell Engager in Advanced Solid Tumors (CTMX-904-101). U.S.National Institutes of Health. | |||||
| REF 90 | ClinicalTrials.gov (NCT02303678) D2C7 for Adult Patients With Recurrent Malignant Glioma. U.S. National Institutes of Health. | |||||
| REF 91 | ClinicalTrials.gov (NCT03638167) EGFR806-specific CAR T Cell Locoregional Immunotherapy for EGFR-positive Recurrent or Refractory Pediatric CNS Tumors | |||||
| REF 92 | Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC). Vaccines (Basel). 2015 Jul 6;3(3):519-43. | |||||
| REF 93 | ClinicalTrials.gov (NCT01963715) A Phase 1 Study of IMGN289 in Adult Patients With EGFR-positive Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 94 | ClinicalTrials.gov (NCT00676299) A Safety and Dose-finding Study of JNJ-26483327, a Drug in Development for Cancer, for Patients With Advanced and/or Refractory Solid Malignancies.. U.S. National Institutes of Health. | |||||
| REF 95 | ClinicalTrials.gov (NCT02221882) A Study of LY3164530 in Participants With Cancer | |||||
| REF 96 | ClinicalTrials.gov (NCT03526835) A Study of Bispecific Antibody MCLA-158 in Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 97 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035447) | |||||
| REF 98 | ClinicalTrials.gov (NCT01009866) Study of Immunotoxin, MR1-1. U.S. National Institutes of Health. | |||||
| REF 99 | ClinicalTrials.gov (NCT02537002) Single Dose Study of PF-05230907 in Healthy Japanese Subjects. | |||||
| REF 100 | ClinicalTrials.gov (NCT04603287) A Study of SI-B001, an EGFR/HER3 Bispecific Antibody, in Locally Advanced or Metastatic Epithelial Tumors. U.S. National Institutes of Health. | |||||
| REF 101 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7642). | |||||
| REF 102 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013689) | |||||
| REF 103 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800029052) | |||||
| REF 104 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024847) | |||||
| REF 105 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005268) | |||||
| REF 106 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6011). | |||||
| REF 107 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019257) | |||||
| REF 108 | A comparison of non-biologically active truncated EGF (EGFt) and full-length hEGF for delivery of Auger electron-emitting 111In to EGFR-positive br... Nucl Med Biol. 2015 Dec;42(12):931-8. | |||||
| REF 109 | Preclinical pharmacokinetic, biodistribution, toxicology, and dosimetry studies of 111In-DTPA-human epidermal growth factor: an auger electron-emitting radiotherapeutic agent for epidermal growth factor receptor-positive breast cancer. J Nucl Med. 2006 Jun;47(6):1023-31. | |||||
| REF 110 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026610) | |||||
| REF 111 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018392) | |||||
| REF 112 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004971) | |||||
| REF 113 | The heparin-binding domain of heparin-binding EGF-like growth factor can target Pseudomonas exotoxin to kill cells exclusively through heparan sulfate proteoglycans. J Cell Sci. 1994 Sep;107 ( Pt 9):2599-608. | |||||
| REF 114 | Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994 Aug 5;269(31):20060-6. | |||||
| REF 115 | Antitumor Activity of Amivantamab (JNJ-61186372), an EGFR-MET Bispecific Antibody, in Diverse Models of EGFR Exon 20 Insertion-Driven NSCLC. Cancer Discov. 2020 Aug;10(8):1194-1209. | |||||
| REF 116 | Boehringer Ingelheim. Product Development Pipeline. June 2 2009. | |||||
| REF 117 | Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002 Oct;29(5 Suppl 14):31-7. | |||||
| REF 118 | Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers. Semin Oncol. 2002 Feb;29(1 Suppl 4):27-36. | |||||
| REF 119 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1797). | |||||
| REF 120 | Quantitative prediction of fold resistance for inhibitors of EGFR. Biochemistry. 2009 Sep 8;48(35):8435-48. | |||||
| REF 121 | Gefitinib ('Iressa', ZD1839) and new epidermal growth factor receptor inhibitors. Br J Cancer. 2004 Feb 9;90(3):566-72. | |||||
| REF 122 | Targeting' the epidermal growth factor receptor tyrosine kinase with gefitinib (Iressa) in non-small cell lung cancer (NSCLC). Semin Cancer Biol. 2004 Feb;14(1):33-40. | |||||
| REF 123 | Triple negative breast cancer--current status and prospective targeted treatment based on HER1 (EGFR), TOP2A and C-MYC gene assessment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009 Mar;153(1):13-7. | |||||
| REF 124 | Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007 Jan;12(1-2):34-42. | |||||
| REF 125 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 126 | Rindopepimut, a 14-mer injectable peptide vaccine against EGFRvIII for the potential treatment of glioblastoma multiforme. Curr Opin Mol Ther. 2010 Dec;12(6):741-54. | |||||
| REF 127 | AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 Sep;4(9):1046-61. | |||||
| REF 128 | Integration of panitumumab into the treatment of colorectal cancer. Crit Rev Oncol Hematol. 2010 Apr;74(1):16-26. | |||||
| REF 129 | Synthesis and Src kinase inhibitory activity of a series of 4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-furyl-3-quinolinecarbonitriles. J Med Chem. 2006 Dec 28;49(26):7868-76. | |||||
| REF 130 | Nasopharyngeal carcinoma: Current treatment options and future directions. J Nasopharyng Carcinoma, 2014, 1(16): e16. | |||||
| REF 131 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||||
| REF 132 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||||
| REF 133 | Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer. 2011 Aug;73(2):195-202. | |||||
| REF 134 | Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial. J Thorac Oncol. 2020 Dec;15(12):1907-1918. | |||||
| REF 135 | ClinicalTrials.gov (NCT04305795) An Open-label Study Using ASP-1929 Photoimmunotherapy in Combination With Anti-PD1 Therapy in EGFR Expressing Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 136 | Clinical pipeline report, company report or official report of Roche (2009). | |||||
| REF 137 | Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015 Apr 30;372(18):1700-9. | |||||
| REF 138 | Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013 Dec;3(12):1404-15. | |||||
| REF 139 | Dual irreversible kinase inhibitors: quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cyst... Bioorg Med Chem. 2007 Jun 1;15(11):3635-48. | |||||
| REF 140 | Sym004, a novel EGFR antibody mixture, can overcome acquired resistance to cetuximab. Neoplasia. 2013 Oct;15(10):1196-206. | |||||
| REF 141 | Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody. Mol Cancer Ther. 2015 May;14(5):1141-51. | |||||
| REF 142 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 143 | Marine-Sourced Anti-Cancer and Cancer Pain Control Agents in Clinical and Late Preclinical Development. Mar Drugs. 2014 January; 12(1): 255-278. | |||||
| REF 144 | Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002 Mar 1;94(5):1593-611. | |||||
| REF 145 | Phase I dose escalation study of ASP8273, a mutant-selective irreversible EGFR inhibitor, in subjects with EGFR mutation positive NSCLC, Journal of Clinical Oncology, Vol 33, No 15_suppl (May 20 Supplement), 2015: 8083. | |||||
| REF 146 | AC480, formerly BMS-599626, a pan Her inhibitor, enhances radiosensitivity and radioresponse of head and neck squamous cell carcinoma cells in vitro and in vivo. Invest New Drugs. 2011 Aug;29(4):554-61. | |||||
| REF 147 | Preclinical pharmacokinetics and in vitro metabolism of BMS-690514, a potent inhibitor of EGFR and VEGFR2. J Pharm Sci. 2010 Aug;99(8):3579-93. | |||||
| REF 148 | Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer. 2012 May 15;130(10):2445-54. | |||||
| REF 149 | Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell. 2008 Apr;13(4):365-73. | |||||
| REF 150 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 151 | EGFR tyrosine kinase inhibitor pelitinib regulates radiation-induced p65-dependent telomerase activation in squamous cell carcinoma.Radiat Res.2013 Mar;179(3):304-12. | |||||
| REF 152 | Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin Cancer Res. 2021 Mar 1;27(5):1463-1475. | |||||
| REF 153 | BiPar Sciences Co-founder Reunites Management Team At TriAct Therapeutics to Advance Clinical Stage Cancer Programs. TriAct Therapeutics. Sept. 10, 2009. | |||||
| REF 154 | Clinical pipeline report, company report or official report of Affimed Therapeutics. | |||||
| REF 155 | Clinical pipeline report, company report or official report of Black Diamond Therapeutics. | |||||
| REF 156 | Clinical pipeline report, company report or official report of EpimAb Biotherapeutics. | |||||
| REF 157 | Regression of EGFR positive established solid tumors in mice with the conditionally active T cell engager TAK-186. J Immunother Cancer. 2022 Jun;10(6):e004336. | |||||
| REF 158 | Clinical pipeline report, company report or official report of Zentalis Pharmaceuticals. | |||||
| REF 159 | AMG 595, an Anti-EGFRvIII Antibody-Drug Conjugate, Induces Potent Antitumor Activity against EGFRvIII-Expressing Glioblastoma. Mol Cancer Ther. 2015 Jul;14(7):1614-24. | |||||
| REF 160 | Company report (Biooncology) | |||||
| REF 161 | AST1306, a novel irreversible inhibitor of the epidermal growth factor receptor 1 and 2, exhibits antitumor activity both in vitro and in vivo. PLoS One. 2011;6(7):e21487. | |||||
| REF 162 | Development and validation of a sensitive LC-MS/MS assay for the simultaneous quantification of allitinib and its two metabolites in human plasma.J Pharm Biomed Anal.2013 Dec;86:49-55. | |||||
| REF 163 | Metabolism and pharmacokinetics of allitinib in cancer patients: the roles of cytochrome P450s and epoxide hydrolase in its biotransformation.Drug Metab Dispos.2014 May;42(5):872-84. | |||||
| REF 164 | Clinical pipeline report, company report or official report of AstraZeneca | |||||
| REF 165 | Phase I and pharmacokinetic study of BIBX 1382 BS, an epidermal growth factor receptor (EGFR) inhibitor, given in a continuous daily oral administration. Eur J Cancer. 2002 May;38(8):1072-80. | |||||
| REF 166 | TAS6417, A Novel EGFR Inhibitor Targeting Exon 20 Insertion Mutations. Mol Cancer Ther. 2018 Aug;17(8):1648-1658. | |||||
| REF 167 | Clinical pipeline report, company report or official report of CytomX | |||||
| REF 168 | Improved efficacy against malignant brain tumors with EGFRwt/EGFRvIII targeting immunotoxin and checkpoint inhibitor combinations. J Immunother Cancer. 2019 May 29;7(1):142. | |||||
| REF 169 | World Antibody-Drug Conjugate Summit, October 15-16, 2013, San Francisco, CA. MAbs. 2014 January 1; 6(1): 18-29. | |||||
| REF 170 | National Cancer Institute Drug Dictionary (drug id 596693). | |||||
| REF 171 | Clinical pipeline report, company report or official report of Merus. | |||||
| REF 172 | A Phase I Study of Immunotoxin, MR1-1. Duke University report. | |||||
| REF 173 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 174 | Clinical pipeline report, company report or official report of SystImmune. | |||||
| REF 175 | Pyrrolo[2,3-d]pyrimidines active as Btk inhibitors.Expert Opin Ther Pat. 2017 Dec;27(12):1305-1318. | |||||
| REF 176 | Epidermal growth factor receptor inhibitor PKI-166 governs cardiovascular protection without beneficial effects on the kidney in hypertensive 5/6 nephrectomized rats.J Pharmacol Exp Ther.2013 Jun;345(3):393-403. | |||||
| REF 177 | GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin Cancer Res. 2013 Mar 1;19(5):1126-38. | |||||
| REF 178 | EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice.Diabetes.2009 Dec;58(12):2910-9. | |||||
| REF 179 | A comparison of EGF and MAb 528 labeled with 111In for imaging human breast cancer. J Nucl Med. 2000 May;41(5):903-11. | |||||
| REF 180 | Clinical pipeline report, company report or official report of Bristol-Myers Squibb. | |||||
| REF 181 | WO patent application no. 2009,0582,67, Benzomorpholine derivatives and methods of use. | |||||
| REF 182 | Retinoic acid-induced RB (retinoblastoma) hypophosphorylation enhanced by CGP 52411 (4,5-dianilinophthalimide), an EGF family tyrosine kinase receptor inhibitor. Eur J Cell Biol. 1996 Apr;69(4):327-34. | |||||
| REF 183 | Dianilinophthalimides: potent and selective, ATP-competitive inhibitors of the EGF-receptor protein tyrosine kinase. J Med Chem. 1994 Apr 1;37(7):1015-27. | |||||
| REF 184 | Tyrosine kinase inhibitors. 5. Synthesis and structure-activity relationships for 4-[(phenylmethyl)amino]- and 4-(phenylamino)quinazolines as poten... J Med Chem. 1995 Sep 1;38(18):3482-7. | |||||
| REF 185 | Tyrosine kinase inhibitors. 9. Synthesis and evaluation of fused tricyclic quinazoline analogues as ATP site inhibitors of the tyrosine kinase acti... J Med Chem. 1996 Feb 16;39(4):918-28. | |||||
| REF 186 | 5-Benzylidene-hydantoins: synthesis and antiproliferative activity on A549 lung cancer cell line. Eur J Med Chem. 2009 Sep;44(9):3471-9. | |||||
| REF 187 | Protein-tyrosine kinase inhibition: mechanism-based discovery of antitumor agents. J Nat Prod. 1992 Nov;55(11):1529-60. | |||||
| REF 188 | Anthraquinones and betaenone derivatives from the sponge-associated fungus Microsphaeropsis species: novel inhibitors of protein kinases. J Nat Prod. 2000 Jun;63(6):739-45. | |||||
| REF 189 | Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine... J Med Chem. 1991 Jun;34(6):1896-907. | |||||
| REF 190 | Design, synthesis, and biological evaluation of 3,4-diarylmaleimides as angiogenesis inhibitors. J Med Chem. 2006 Feb 23;49(4):1271-81. | |||||
| REF 191 | Use of a pharmacophore model for the design of EGFR tyrosine kinase inhibitors: isoflavones and 3-phenyl-4(1H)-quinolones. J Med Chem. 1999 Mar 25;42(6):1018-26. | |||||
| REF 192 | 5,7-Dimethoxy-3-(4-pyridinyl)quinoline is a potent and selective inhibitor of human vascular beta-type platelet-derived growth factor receptor tyro... J Med Chem. 1994 Aug 19;37(17):2627-9. | |||||
| REF 193 | A novel series of 4-phenoxyquinolines: potent and highly selective inhibitors of PDGF receptor autophosphorylation, Bioorg. Med. Chem. Lett. 7(23):2935-2940 (1997). | |||||
| REF 194 | Syntheses of 4-(indole-3-yl)quinazolines: a new class of epidermal growth factor receptor tyrosine kinase inhibitors. Eur J Med Chem. 2008 Jul;43(7):1478-88. | |||||
| REF 195 | Acryloylamino-salicylanilides as EGFR PTK inhibitors. Bioorg Med Chem Lett. 2006 Jan 15;16(2):469-72. | |||||
| REF 196 | Synthesis and inhibitory activity of 4-alkynyl and 4-alkenylquinazolines: identification of new scaffolds for potent EGFR tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2007 Nov 1;17(21):5863-7. | |||||
| REF 197 | Indazolylamino quinazolines and pyridopyrimidines as inhibitors of the EGFr and C-erbB-2. Bioorg Med Chem Lett. 2001 Jun 4;11(11):1401-5. | |||||
| REF 198 | Computational studies of epidermal growth factor receptor: docking reliability, three-dimensional quantitative structure-activity relationship anal... J Med Chem. 2009 Feb 26;52(4):964-75. | |||||
| REF 199 | Discovery and SAR of 6-substituted-4-anilinoquinazolines as non-competitive antagonists of mGlu5. Bioorg Med Chem Lett. 2009 Dec 1;19(23):6623-6. | |||||
| REF 200 | Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344-52. | |||||
| REF 201 | Cochliobolic acid, a novel metabolite produced by Cochliobolus lunatus, inhibits binding of TGF-alpha to the EGF receptor in a SPA assay. J Nat Prod. 1997 Jan;60(1):6-8. | |||||
| REF 202 | A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20523-8. | |||||
| REF 203 | Tyrosine kinase inhibitors. 19. 6-Alkynamides of 4-anilinoquinazolines and 4-anilinopyrido[3,4-d]pyrimidines as irreversible inhibitors of the erbB... J Med Chem. 2006 Feb 23;49(4):1475-85. | |||||
| REF 204 | Biochemical and cellular effects of c-Src kinase-selective pyrido[2, 3-d]pyrimidine tyrosine kinase inhibitors. Biochem Pharmacol. 2000 Oct 1;60(7):885-98. | |||||
| REF 205 | Novel antiproliferative agents derived from lavendustin A. J Med Chem. 1994 Nov 25;37(24):4079-84. | |||||
| REF 206 | Effects of hEGF (MG111) on gastric mucosal potential difference in rats. Scand J Gastroenterol Suppl. 1989;162:198-201. | |||||
| REF 207 | Synthesis of a prodrug designed to release multiple inhibitors of the epidermal growth factor receptor tyrosine kinase and an alkylating agent: a n... J Med Chem. 2003 Dec 4;46(25):5546-51. | |||||
| REF 208 | Tyrosine kinase inhibitors. 10. Isomeric 4-[(3-bromophenyl)amino]pyrido[d]-pyrimidines are potent ATP binding site inhibitors of the tyrosine kinas... J Med Chem. 1996 Apr 26;39(9):1823-35. | |||||
| REF 209 | Structure-guided development of affinity probes for tyrosine kinases using chemical genetics. Nat Chem Biol. 2007 Apr;3(4):229-38. | |||||
| REF 210 | Tyrosine kinase inhibitors. 15. 4-(Phenylamino)quinazoline and 4-(phenylamino)pyrido[d]pyrimidine acrylamides as irreversible inhibitors of the ATP... J Med Chem. 1999 May 20;42(10):1803-15. | |||||
| REF 211 | Novel nitrogen mustard-armed combi-molecules for the selective targeting of epidermal growth factor receptor overexperessing solid tumors: discover... J Med Chem. 2007 May 31;50(11):2605-8. | |||||
| REF 212 | The combi-targeting concept: synthesis of stable nitrosoureas designed to inhibit the epidermal growth factor receptor (EGFR). J Med Chem. 2006 Jun 15;49(12):3544-52. | |||||
| REF 213 | Selected novel anticancer treatments targeting cell signaling proteins. Oncologist. 2001;6(6):517-37. | |||||
| REF 214 | Growth factors and their receptors: new targets for prostate cancer therapy. Urology. 2001 Aug;58(2 Suppl 1):114-22. | |||||
| REF 215 | Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor... J Med Chem. 2005 Feb 24;48(4):1107-31. | |||||
| REF 216 | Discovery of a series of 2,5-diaminopyrimidine covalent irreversible inhibitors of Bruton's tyrosine kinase with in vivo antitumor activity. J Med Chem. 2014 Jun 26;57(12):5112-28. | |||||
| REF 217 | Tyrosine kinase inhibitors. 8. An unusually steep structure-activity relationship for analogues of 4-(3-bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a potent inhibitor of the epidermal growth factor receptor. J Med Chem. 1996 Jan 5;39(1):267-76. | |||||
| REF 218 | Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008 Nov;4(11):691-9. | |||||
| REF 219 | Biological evaluation of a multi-targeted small molecule inhibitor of tumor-induced angiogenesis. Bioorg Med Chem Lett. 2006 Apr 1;16(7):1950-3. | |||||
| REF 220 | The specificity of JAK3 kinase inhibitors. Blood. 2008 Feb 15;111(4):2155-7. | |||||
| REF 221 | Clinical pipeline report, company report or official report of Biocytogen | |||||
| REF 222 | A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004 Sep 15;64(18):6652-9. | |||||
| REF 223 | Abstract 4451: Evaluation of the therapeutic potential of phosphine oxide pyrazole inhibitors in tumors harboring EGFR C797S mutation. doi:10.1158/1538-7445.AM2019-4451. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

