Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T07303
(Former ID: TTDC00290)
|
|||||
| Target Name |
Vascular endothelial growth factor receptor 3 (FLT-4)
|
|||||
| Synonyms |
VEGFR3; VEGFR-3; VEGF-3 receptor; Tyrosine-protein kinase receptor FLT4; Fms-like tyrosine kinase 4
Click to Show/Hide
|
|||||
| Gene Name |
FLT4
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Renal cell carcinoma [ICD-11: 2C90] | |||||
| Function |
Promotes proliferation, survival and migration of endothelial cells, and regulates angiogenic sprouting. Signaling by activated FLT4 leads to enhanced production of VEGFC, and to a lesser degree VEGFA, thereby creating a positive feedback loop that enhances FLT4 signaling. Modulates KDR signaling by forming heterodimers. The secreted isoform 3 may function as a decoy receptor for VEGFC and/or VEGFD and play an important role as a negative regulator of VEGFC-mediated lymphangiogenesis and angiogenesis. Binding of vascular growth factors to isoform 1 or isoform 2 leads to the activation of several signaling cascades; isoform 2 seems to be less efficient in signal transduction, because it has a truncated C-terminus and therefore lacks several phosphorylation sites. Mediates activation of the MAPK1/ERK2, MAPK3/ERK1 signaling pathway, of MAPK8 and the JUN signaling pathway, and of the AKT1 signaling pathway. Phosphorylates SHC1. Mediates phosphorylation of PIK3R1, the regulatory subunit of phosphatidylinositol 3-kinase. Promotes phosphorylation of MAPK8 at 'Thr-183' and 'Tyr-185', and of AKT1 at 'Ser-473'. Tyrosine-protein kinase that acts as a cell-surface receptor for VEGFC and VEGFD, and plays an essential role in adult lymphangiogenesis and in the development of the vascular network and the cardiovascular system during embryonic development.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MQRGAALCLRLWLCLGLLDGLVSGYSMTPPTLNITEESHVIDTGDSLSISCRGQHPLEWA
WPGAQEAPATGDKDSEDTGVVRDCEGTDARPYCKVLLLHEVHANDTGSYVCYYKYIKARI EGTTAASSYVFVRDFEQPFINKPDTLLVNRKDAMWVPCLVSIPGLNVTLRSQSSVLWPDG QEVVWDDRRGMLVSTPLLHDALYLQCETTWGDQDFLSNPFLVHITGNELYDIQLLPRKSL ELLVGEKLVLNCTVWAEFNSGVTFDWDYPGKQAERGKWVPERRSQQTHTELSSILTIHNV SQHDLGSYVCKANNGIQRFRESTEVIVHENPFISVEWLKGPILEATAGDELVKLPVKLAA YPPPEFQWYKDGKALSGRHSPHALVLKEVTEASTGTYTLALWNSAAGLRRNISLELVVNV PPQIHEKEASSPSIYSRHSRQALTCTAYGVPLPLSIQWHWRPWTPCKMFAQRSLRRRQQQ DLMPQCRDWRAVTTQDAVNPIESLDTWTEFVEGKNKTVSKLVIQNANVSAMYKCVVSNKV GQDERLIYFYVTTIPDGFTIESKPSEELLEGQPVLLSCQADSYKYEHLRWYRLNLSTLHD AHGNPLLLDCKNVHLFATPLAASLEEVAPGARHATLSLSIPRVAPEHEGHYVCEVQDRRS HDKHCHKKYLSVQALEAPRLTQNLTDLLVNVSDSLEMQCLVAGAHAPSIVWYKDERLLEE KSGVDLADSNQKLSIQRVREEDAGRYLCSVCNAKGCVNSSASVAVEGSEDKGSMEIVILV GTGVIAVFFWVLLLLIFCNMRRPAHADIKTGYLSIIMDPGEVPLEEQCEYLSYDASQWEF PRERLHLGRVLGYGAFGKVVEASAFGIHKGSSCDTVAVKMLKEGATASEHRALMSELKIL IHIGNHLNVVNLLGACTKPQGPLMVIVEFCKYGNLSNFLRAKRDAFSPCAEKSPEQRGRF RAMVELARLDRRRPGSSDRVLFARFSKTEGGARRASPDQEAEDLWLSPLTMEDLVCYSFQ VARGMEFLASRKCIHRDLAARNILLSESDVVKICDFGLARDIYKDPDYVRKGSARLPLKW MAPESIFDKVYTTQSDVWSFGVLLWEIFSLGASPYPGVQINEEFCQRLRDGTRMRAPELA TPAIRRIMLNCWSGDPKARPAFSELVEILGDLLQGRGLQEEEEVCMAPRSSQSSEEGSFS QVSTMALHIAQADAEDSPPSLQRHSLAARYYNWVSFPGCLARGAETRGSSRMKTFEEFPM TPTTYKGSVDNQTDSGMVLASEEFEQIESRHRQESGFSCKGPGQNVAVTRAHPDSQGRRR RPERGARGGQVFYNSEYGELSEPSEEDHCSPSARVTFFTDNSY Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T29JZ3 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Tivozanib | Drug Info | Approved | Renal cell carcinoma | [2] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | ABT-869 | Drug Info | Phase 3 | Solid tumour/cancer | [3], [4], [5] | |
| 2 | E-3810 | Drug Info | Phase 3 | Solid tumour/cancer | [6], [7] | |
| 3 | PI-88 | Drug Info | Phase 3 | Hepatocellular carcinoma | [8] | |
| 4 | Sulfatinib | Drug Info | Phase 3 | Neuroendocrine cancer | [9] | |
| 5 | Taberminogene vadenovec | Drug Info | Phase 3 | Vascular restinosis | [10] | |
| 6 | MGCD516 | Drug Info | Phase 2/3 | Solid tumour/cancer | [11] | |
| 7 | Famitinib | Drug Info | Phase 2 | Solid tumour/cancer | [12], [13] | |
| 8 | VATALANIB | Drug Info | Phase 2 | Solid tumour/cancer | [14], [15] | |
| 9 | MK-2461 | Drug Info | Phase 1/2 | Alzheimer disease | [16] | |
| 10 | IMC-3C5 | Drug Info | Phase 1 | Solid tumour/cancer | [17] | |
| 11 | JNJ-26483327 | Drug Info | Phase 1 | Solid tumour/cancer | [18] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 5 Modulator drugs | + | ||||
| 1 | Tivozanib | Drug Info | [2] | |||
| 2 | ABT-869 | Drug Info | [4], [19] | |||
| 3 | PI-88 | Drug Info | [1] | |||
| 4 | Taberminogene vadenovec | Drug Info | [22] | |||
| 5 | IMC-3C5 | Drug Info | [25] | |||
| Inhibitor | [+] 28 Inhibitor drugs | + | ||||
| 1 | E-3810 | Drug Info | [20] | |||
| 2 | Sulfatinib | Drug Info | [21] | |||
| 3 | MGCD516 | Drug Info | [23] | |||
| 4 | Famitinib | Drug Info | [13] | |||
| 5 | VATALANIB | Drug Info | [24] | |||
| 6 | MK-2461 | Drug Info | [16] | |||
| 7 | JNJ-26483327 | Drug Info | [26] | |||
| 8 | Pyrimidine derivative 15 | Drug Info | [27] | |||
| 9 | (2-Methoxy-phenyl)-(5-phenyl-oxazol-2-yl)-amine | Drug Info | [24] | |||
| 10 | (5-Phenyl-oxazol-2-yl)-m-tolyl-amine | Drug Info | [24] | |||
| 11 | 2-(5-Phenyl-oxazol-2-ylamino)-benzonitrile | Drug Info | [24] | |||
| 12 | 3,6-Di-pyridin-4-yl-pyrazolo[1,5-a]pyrimidine | Drug Info | [28] | |||
| 13 | 3-(5-Phenyl-oxazol-2-ylamino)-benzonitrile | Drug Info | [24] | |||
| 14 | 4-(5-Phenyl-oxazol-2-ylamino)-benzenesulfonamide | Drug Info | [24] | |||
| 15 | 4-Chloro-N-(2-chloro-benzoyl)-benzenesulfonamide | Drug Info | [29] | |||
| 16 | 4-Chloro-N-(2-methyl-benzoyl)-benzenesulfonamide | Drug Info | [29] | |||
| 17 | 4-Chloro-N-(3-chloro-benzoyl)-benzenesulfonamide | Drug Info | [29] | |||
| 18 | 4-Chloro-N-(4-chloro-benzoyl)-benzenesulfonamide | Drug Info | [29] | |||
| 19 | 4-Chloro-N-(4-nitro-benzoyl)-benzenesulfonamide | Drug Info | [29] | |||
| 20 | Anti-VEGFR 3 mab | Drug Info | [23] | |||
| 21 | CB-676475 | Drug Info | [30] | |||
| 22 | CEP-6331 | Drug Info | [31] | |||
| 23 | N-(2,4-Dichloro-benzoyl)-benzenesulfonamide | Drug Info | [29] | |||
| 24 | N-(3-Bromo-benzoyl)-4-chloro-benzenesulfonamide | Drug Info | [29] | |||
| 25 | Phenyl-(5-phenyl-oxazol-2-yl)-amine | Drug Info | [24] | |||
| 26 | PMID22765894C8h | Drug Info | [32] | |||
| 27 | SAR-131675 | Drug Info | [23] | |||
| 28 | [3-(5-Phenyl-oxazol-2-ylamino)-phenyl]-methanol | Drug Info | [24] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Rap1 signaling pathway | hsa04015 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
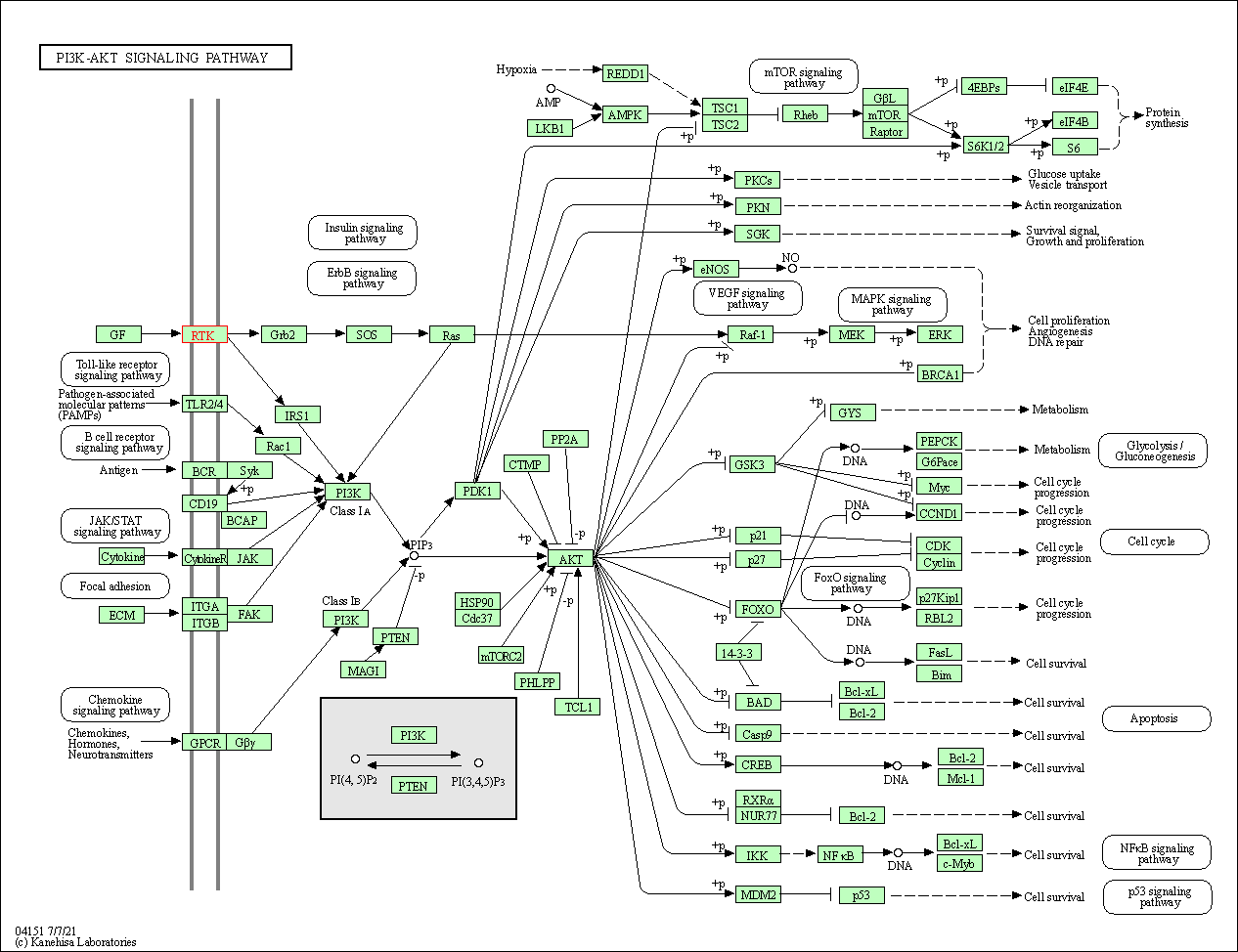
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
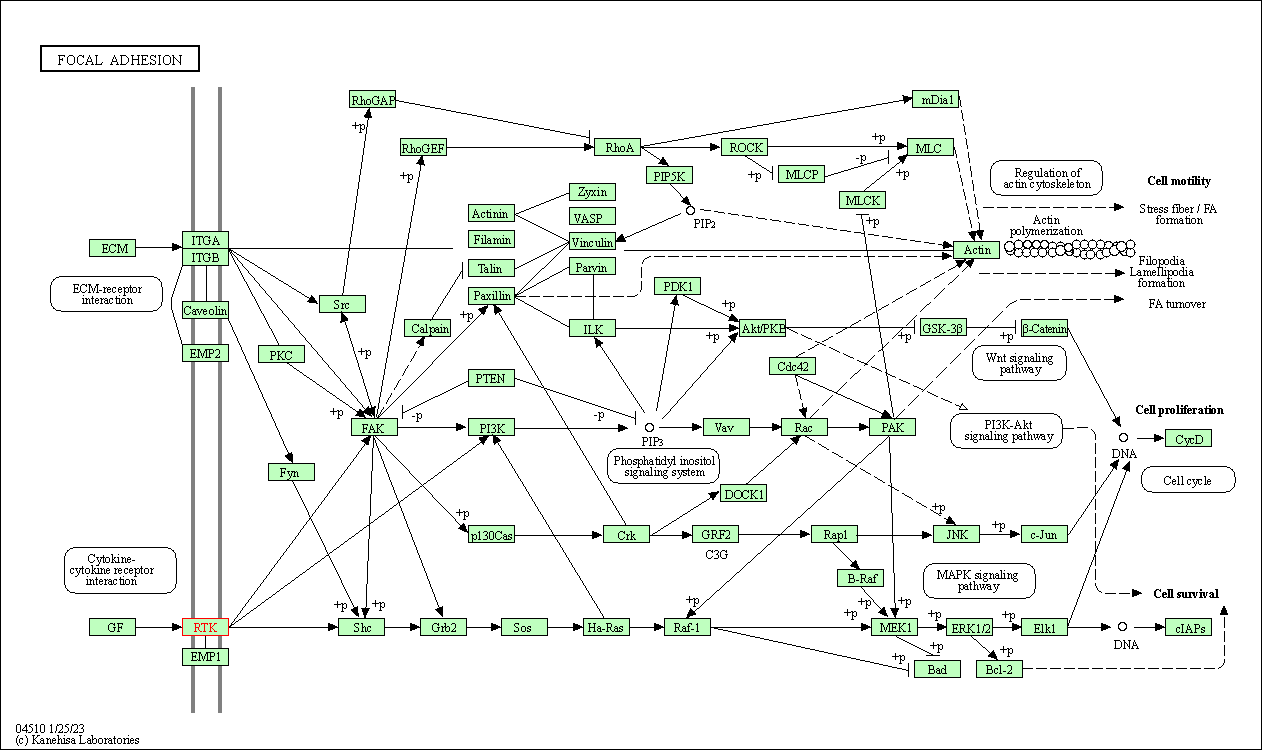
| Focal adhesion | hsa04510 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 14 | Degree centrality | 1.50E-03 | Betweenness centrality | 1.92E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.29E-01 | Radiality | 1.40E+01 | Clustering coefficient | 3.30E-01 |
| Neighborhood connectivity | 4.41E+01 | Topological coefficient | 1.30E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Ras signaling pathway | |||||
| 2 | Rap1 signaling pathway | |||||
| 3 | Cytokine-cytokine receptor interaction | |||||
| 4 | PI3K-Akt signaling pathway | |||||
| 5 | Focal adhesion | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TNFalpha Signaling Pathway | |||||
| PID Pathway | [+] 2 PID Pathways | + | ||||
| 1 | VEGF and VEGFR signaling network | |||||
| 2 | VEGFR3 signaling in lymphatic endothelium | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | VEGF binds to VEGFR leading to receptor dimerization | |||||
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | Signaling by VEGF | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Company report (Medigen) | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5657). | |||||
| REF 4 | Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006 Apr;5(4):995-1006. | |||||
| REF 5 | Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors. Mol Cancer Ther. 2006 Apr;5(4):1007-13. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7649). | |||||
| REF 7 | ClinicalTrials.gov (NCT02135107) A Double-blind Comparative Study of the Efficacy and Safety of E3810 10mg Once and Twice Daily in Maintenance Therapy for PPI Resistant Gastroesophageal Reflux Disease Patients. U.S. National Institutes of Health. | |||||
| REF 8 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||||
| REF 9 | ClinicalTrials.gov (NCT02589821) Phase III Study of Surufatinib in Treating Advanced Pancreatic Neuroendocrine Tumors. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT00895479) Adenovirus Vascular Endothelial Growth Factor (VEGF) Therapy in Vascular Access - Novel Trinam AGainst Control Evidence. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT04887870) Study of Sitravatinib With or Without Other Anticancer Therapies Receiving Clinical Benefit From Parent Study. U.S. National Institutes of Health. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7886). | |||||
| REF 13 | Metabolism and bioactivation of famitinib, a novel inhibitor of receptor tyrosine kinase, in cancer patients. Br J Pharmacol. 2013 Apr;168(7):1687-706. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5705). | |||||
| REF 15 | ClinicalTrials.gov (NCT00348790) Vatalanib in Treating Patients With Recurrent or Progressive Meningioma. U.S. National Institutes of Health. | |||||
| REF 16 | MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 2010 Feb 15;70(4):1524-33. | |||||
| REF 17 | ClinicalTrials.gov (NCT01288989) A Study of Anti-VEGFR-3 Monoclonal Antibody IMC-3C5 in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT00676299) A Safety and Dose-finding Study of JNJ-26483327, a Drug in Development for Cancer, for Patients With Advanced and/or Refractory Solid Malignancies.. U.S. National Institutes of Health. | |||||
| REF 19 | Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors. Mol Cancer Ther. 2006 Apr;5(4):1007-13. | |||||
| REF 20 | E-3810 is a potent dual inhibitor of VEGFR and FGFR that exerts antitumor activity in multiple preclinical models. Cancer Res. 2011 Feb 15;71(4):1396-405. | |||||
| REF 21 | Clinical pipeline report, company report or official report of Hutchison Medi Pharma. | |||||
| REF 22 | Advances in kinase targeting: current clinical use and clinical trials. Trends Pharmacol Sci. 2014 Nov;35(11):604-20. | |||||
| REF 23 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1814). | |||||
| REF 24 | Discovery and evaluation of 2-anilino-5-aryloxazoles as a novel class of VEGFR2 kinase inhibitors. J Med Chem. 2005 Mar 10;48(5):1610-9. | |||||
| REF 25 | Bevacizumab and breast cancer: what does the future hold . Future Oncol. 2012 April; 8(4): 403-414. | |||||
| REF 26 | National Cancer Institute Drug Dictionary (drug id 596693). | |||||
| REF 27 | VEGFR-2 inhibitors and the therapeutic applications thereof: a patent review (2012-2016).Expert Opin Ther Pat. 2017 Sep;27(9):987-1004. | |||||
| REF 28 | Synthesis and initial SAR studies of 3,6-disubstituted pyrazolo[1,5-a]pyrimidines: a new class of KDR kinase inhibitors. Bioorg Med Chem Lett. 2002 Oct 7;12(19):2767-70. | |||||
| REF 29 | Acyl sulfonamide anti-proliferatives: benzene substituent structure-activity relationships for a novel class of antitumor agents. J Med Chem. 2004 Oct 21;47(22):5367-80. | |||||
| REF 30 | Synthesis of a novel biotin-tagged photoaffinity probe for VEGF receptor tyrosine kinases. Bioorg Med Chem Lett. 2006 Jan 1;16(1):129-33. | |||||
| REF 31 | Mixed-lineage kinase 1 and mixed-lineage kinase 3 subtype-selective dihydronaphthyl[3,4-a]pyrrolo[3,4-c]carbazole-5-ones: optimization, mixed-linea... J Med Chem. 2008 Sep 25;51(18):5680-9. | |||||
| REF 32 | The design, synthesis, and biological evaluation of potent receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2012 Aug 1;22(15):4979-85. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

