Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T10877
(Former ID: TTDNC00424)
|
|||||
| Target Name |
Cyclic ADP-ribose hydrolase 1 (CD38)
|
|||||
| Synonyms |
cADPr hydrolase 1; T10; Cyclic ADPribose hydrolase 1; ADPribosyl cyclase 1; ADPRC 1; ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1; ADP-ribosyl cyclase 1; 2'-phospho-cyclic-ADP-ribose transferase; 2'-phospho-ADP-ribosyl cyclase/2'-phospho-cyclic-ADP-ribose transferase; 2'-phospho-ADP-ribosyl cyclase
Click to Show/Hide
|
|||||
| Gene Name |
CD38
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Multiple myeloma [ICD-11: 2A83] | |||||
| Function |
Has cADPr hydrolase activity. Also moonlights as a receptor in cells of the immune system. Synthesizes the second messagers cyclic ADP-ribose and nicotinate-adenine dinucleotide phosphate, the former a second messenger for glucose-induced insulin secretion.
Click to Show/Hide
|
|||||
| BioChemical Class |
Glycosylase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.2.2.6
|
|||||
| Sequence |
MANCEFSPVSGDKPCCRLSRRAQLCLGVSILVLILVVVLAVVVPRWRQQWSGPGTTKRFP
ETVLARCVKYTEIHPEMRHVDCQSVWDAFKGAFISKHPCNITEEDYQPLMKLGTQTVPCN KILLWSRIKDLAHQFTQVQRDMFTLEDTLLGYLADDLTWCGEFNTSKINYQSCPDWRKDC SNNPVSVFWKTVSRRFAEAACDVVHVMLNGSRSKIFDKNSTFGSVEVHNLQPEKVQTLEA WVIHGGREDSRDLCQDPTIKELESIISKRNIQFSCKNIYRPDKFLQCVKNPEDSSCTSEI Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T86MX7 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Daratumumab | Drug Info | Approved | Multiple myeloma | [2], [3], [4] | |
| Clinical Trial Drug(s) | [+] 22 Clinical Trial Drugs | + | ||||
| 1 | Mezagitamab | Drug Info | Phase 2 | Myasthenia gravis | [5] | |
| 2 | 4SCAR19 and 4SCAR38 | Drug Info | Phase 1/2 | B-cell lymphoma | [6] | |
| 3 | Anti-CD38 CAR-T cells | Drug Info | Phase 1/2 | Multiple myeloma | [7] | |
| 4 | CD38 and BCMA CAR-T Cells | Drug Info | Phase 1/2 | Multiple myeloma | [8] | |
| 5 | CD38 CAR T cells | Drug Info | Phase 1/2 | Multiple myeloma | [9] | |
| 6 | CD38 CAR-T Cell | Drug Info | Phase 1/2 | Acute lymphoblastic leukaemia | [10] | |
| 7 | CD38-specific gene-engineered T cells | Drug Info | Phase 1/2 | Acute myeloid leukaemia | [11] | |
| 8 | ISB 1342 | Drug Info | Phase 1/2 | Multiple myeloma | [12] | |
| 9 | MOR-202 | Drug Info | Phase 1/2 | Multiple myeloma | [13] | |
| 10 | SAR-650984 | Drug Info | Phase 1/2 | Haematological malignancy | [14] | |
| 11 | 225Ac-labelled aCD38 | Drug Info | Phase 1 | Multiple myeloma | [15] | |
| 12 | AMG 424 | Drug Info | Phase 1 | Multiple myeloma | [16] | |
| 13 | Anti-CD38 CAR-T cells | Drug Info | Phase 1 | Multiple myeloma | [17] | |
| 14 | CART-38 cells | Drug Info | Phase 1 | Myelodysplastic syndrome | [18] | |
| 15 | CD38 CAR-T | Drug Info | Phase 1 | Multiple myeloma | [19] | |
| 16 | KP1237 | Drug Info | Phase 1 | Multiple myeloma | [20] | |
| 17 | SAR442085 | Drug Info | Phase 1 | Plasma cell myeloma | [21] | |
| 18 | SAR442257 | Drug Info | Phase 1 | Malignant neoplasm | [22] | |
| 19 | SAR444559 | Drug Info | Phase 1 | Inflammation | [23] | |
| 20 | TAK-169 | Drug Info | Phase 1 | Multiple myeloma | [24] | |
| 21 | TAK-573 | Drug Info | Phase 1 | Refractory multiple myeloma | [25] | |
| 22 | CAR-T cells targeting CD38 | Drug Info | Clinical trial | Multiple myeloma | [26] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| CAR-T-Cell-Therapy(Dual specific) | [+] 2 CAR-T-Cell-Therapy(Dual specific) drugs | + | ||||
| 1 | 4SCAR19 and 4SCAR38 | Drug Info | [6] | |||
| 2 | CD38 and BCMA CAR-T Cells | Drug Info | [8] | |||
| CAR-T-Cell-Therapy | [+] 7 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Anti-CD38 CAR-T cells | Drug Info | [7] | |||
| 2 | CD38 CAR T cells | Drug Info | [9] | |||
| 3 | CD38 CAR-T Cell | Drug Info | [10] | |||
| 4 | CD38-specific gene-engineered T cells | Drug Info | [11] | |||
| 5 | Anti-CD38 CAR-T cells | Drug Info | [17] | |||
| 6 | CART-38 cells | Drug Info | [18] | |||
| 7 | CAR-T cells targeting CD38 | Drug Info | [26], [36] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | ISB 1342 | Drug Info | [28] | |||
| 2 | AMG 424 | Drug Info | [32] | |||
| 3 | SAR442085 | Drug Info | [33] | |||
| 4 | SAR442257 | Drug Info | [33] | |||
| 5 | TAK-169 | Drug Info | [34] | |||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | HuMax-CD38b | Drug Info | [37] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Nicotinamide | Ligand Info | |||||
| Structure Description | Crystal structure of human CD38 extracellular domain, ara-F-ribose-5'-phosphate/nicotinamide complex | PDB:3DZG | ||||
| Method | X-ray diffraction | Resolution | 1.65 Å | Mutation | Yes | [38] |
| PDB Sequence |
RWRQTWSGPG
54 TTKRFPETVL64 ARCVKYTEIH74 PEMRHVDCQS84 VWDAFKGAFI94 SKHPCDITEE 104 DYQPLMKLGT114 QTVPCNKILL124 WSRIKDLAHQ134 FTQVQRDMFT144 LEDTLLGYLA 154 DDLTWCGEFD164 TSKINYQSCP174 DWRKDCSNNP184 VSVFWKTVSR194 RFAEAACDVV 204 HVMLDGSRSK214 IFDKDSTFGS224 VEVHNLQPEK234 VQTLEAWVIH244 GGREDSRDLC 254 QDPTIKELES264 IISKRNIQFS274 CKNIYRPDKF284 LQCVKNPEDS294 SC |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Guanosine-5'-Triphosphate | Ligand Info | |||||
| Structure Description | Crystal structure of human CD38 extracellular domain, GTP complex | PDB:3DZH | ||||
| Method | X-ray diffraction | Resolution | 1.60 Å | Mutation | Yes | [38] |
| PDB Sequence |
RWRQTWSGPG
54 TTKRFPETVL64 ARCVKYTEIH74 PEMRHVDCQS84 VWDAFKGAFI94 SKHPCDITEE 104 DYQPLMKLGT114 QTVPCNKILL124 WSRIKDLAHQ134 FTQVQRDMFT144 LEDTLLGYLA 154 DDLTWCGEFD164 TSKINYQSCP174 DWRKDCSNNP184 VSVFWKTVSR194 RFAEAACDVV 204 HVMLDGSRSK214 IFDKDSTFGS224 VEVHNLQPEK234 VQTLEAWVIH244 GGREDSRDLC 254 QDPTIKELES264 IISKRNIQFS274 CKNIYRPDKF284 LQCVKNPEDS294 SC |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|

| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Nicotinate and nicotinamide metabolism | hsa00760 | Affiliated Target |

|
| Class: Metabolism => Metabolism of cofactors and vitamins | Pathway Hierarchy | ||
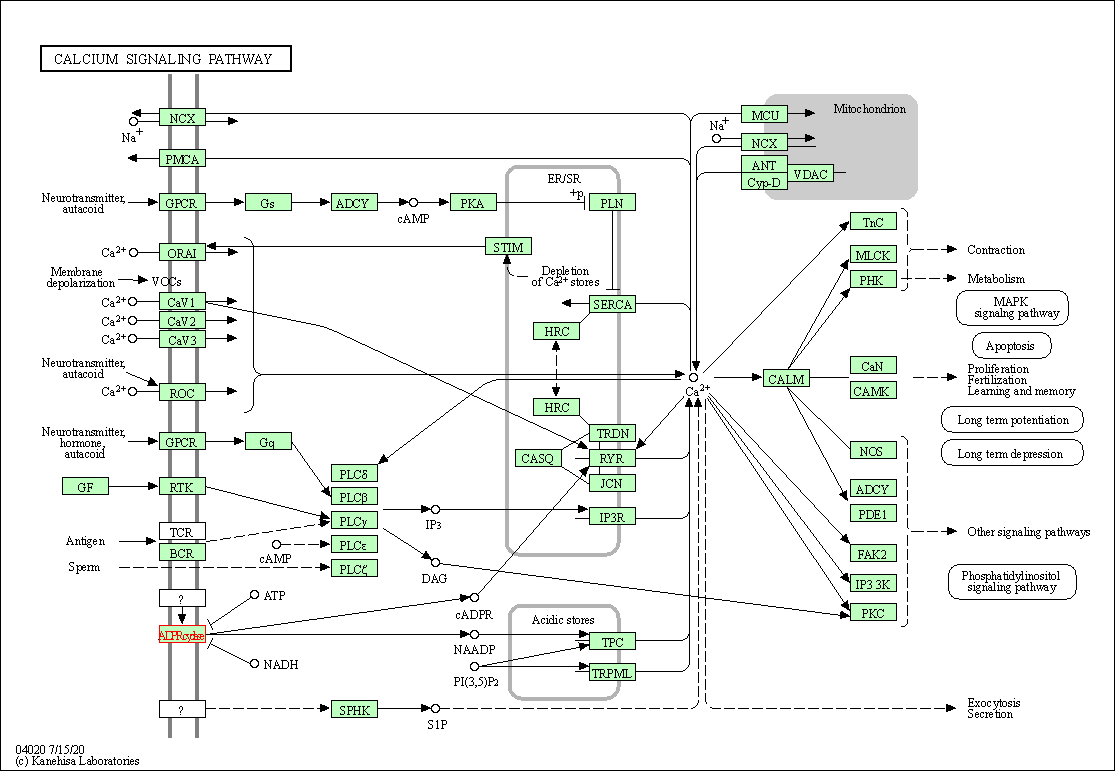
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
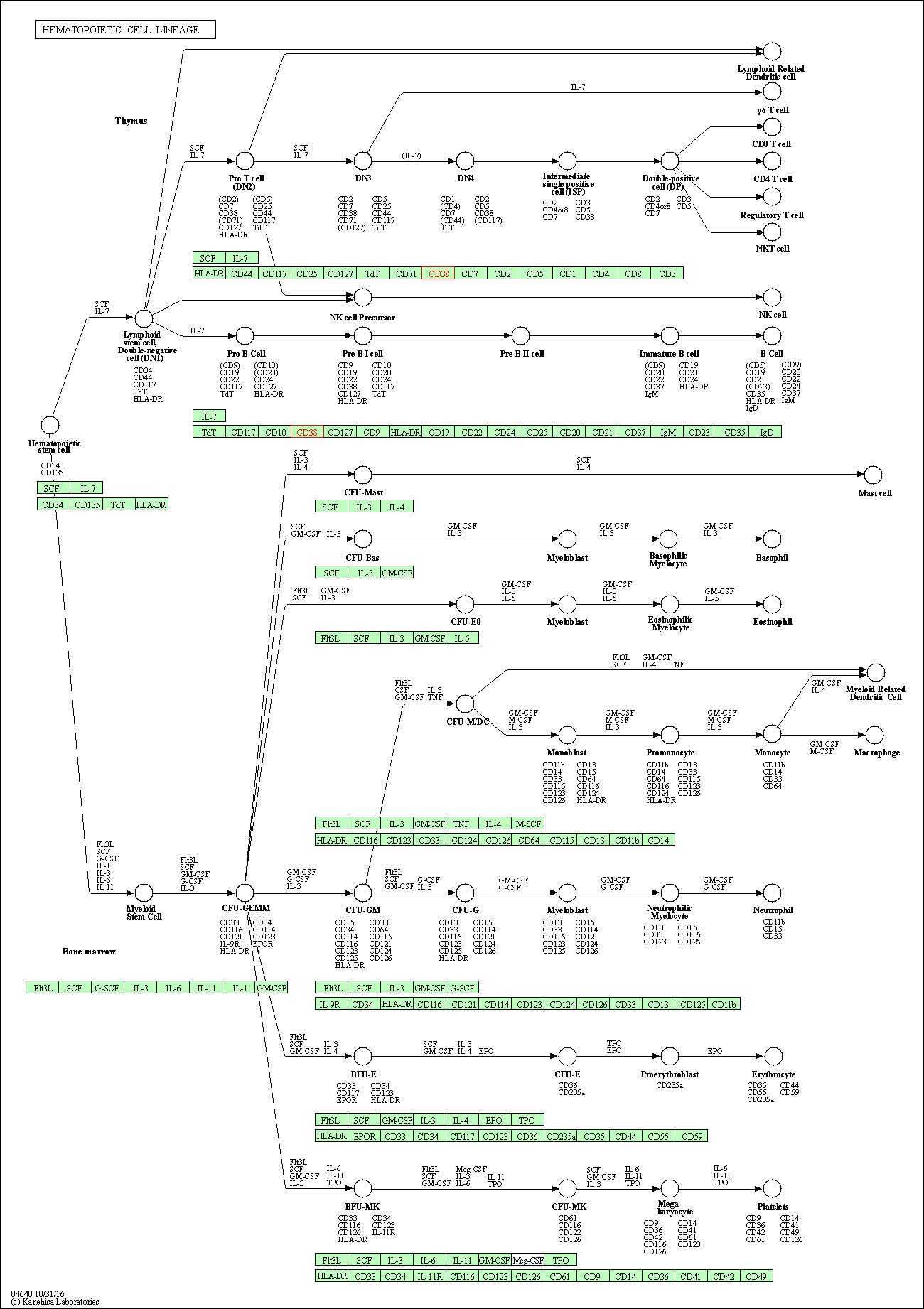
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
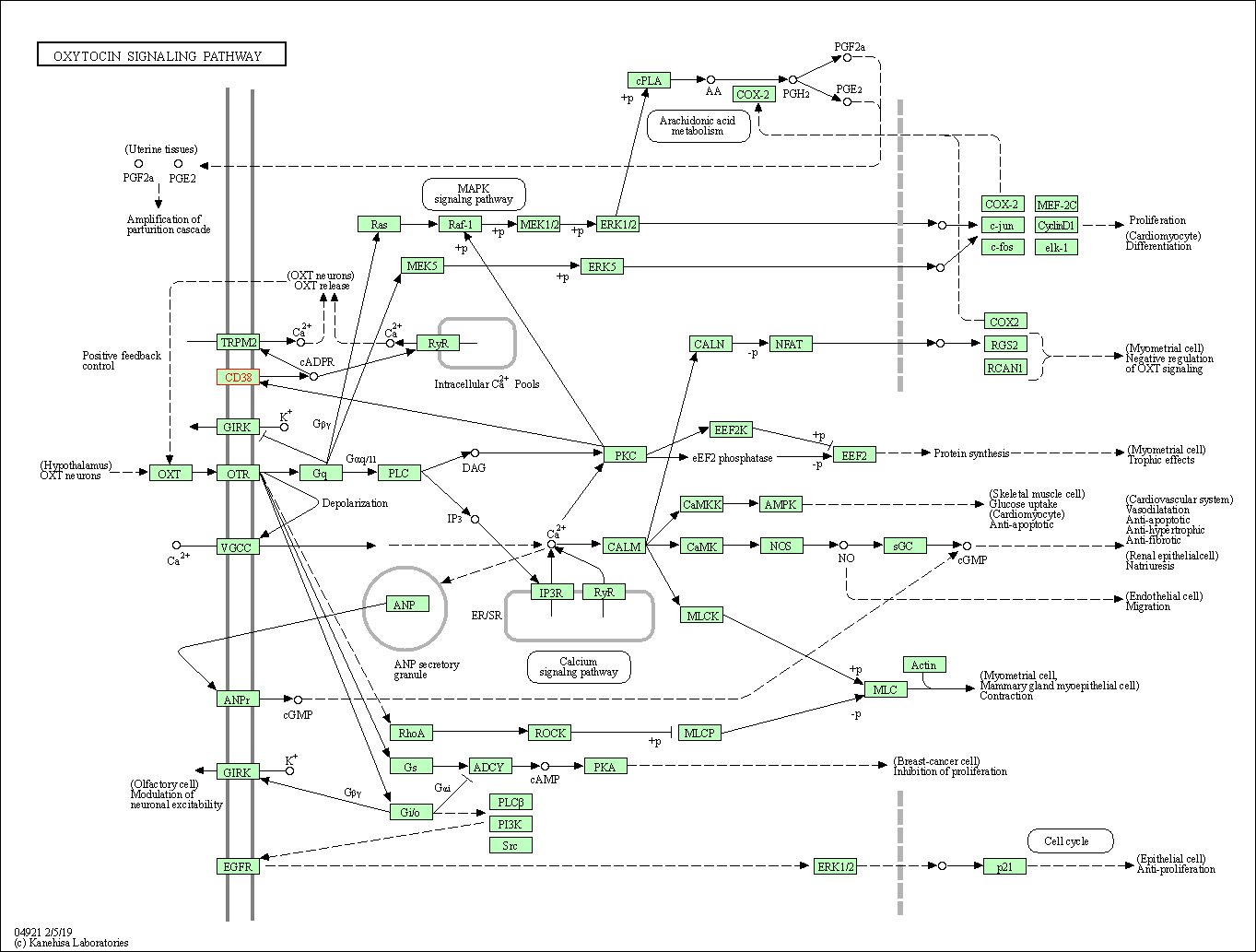
| Oxytocin signaling pathway | hsa04921 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
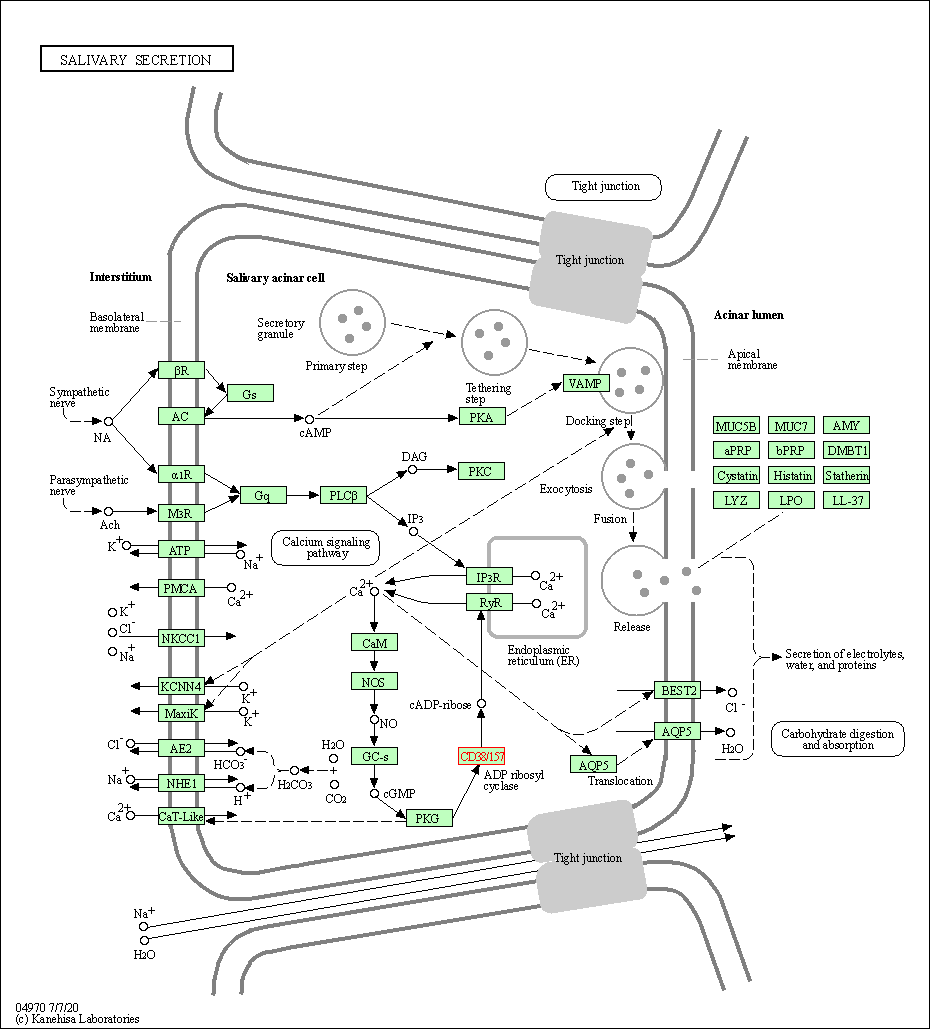
| Salivary secretion | hsa04970 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Pancreatic secretion | hsa04972 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 5.65E-07 |
|---|---|---|---|---|---|
| Closeness centrality | 2.06E-01 | Radiality | 1.36E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 4.60E+01 | Topological coefficient | 5.29E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 8 KEGG Pathways | + | ||||
| 1 | Nicotinate and nicotinamide metabolism | |||||
| 2 | Metabolic pathways | |||||
| 3 | Calcium signaling pathway | |||||
| 4 | Hematopoietic cell lineage | |||||
| 5 | Oxytocin signaling pathway | |||||
| 6 | Salivary secretion | |||||
| 7 | Pancreatic secretion | |||||
| 8 | Epstein-Barr virus infection | |||||
| NetPath Pathway | [+] 2 NetPath Pathways | + | ||||
| 1 | Leptin Signaling Pathway | |||||
| 2 | TCR Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | CCKR signaling map ST | |||||
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | Oxytocin signaling | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015 Sep 24;373(13):1207-19. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7395). | |||||
| REF 3 | ClinicalTrials.gov (NCT02252172) Study Comparing Daratumumab, Lenalidomide, and Dexamethasone With Lenalidomide and Dexamethasone in Participants With Previously Untreated Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 5 | ClinicalTrials.gov (NCT04159805) A Phase 2, Randomized, Placebo-Controlled Study to Evaluate Safety, Tolerability, and Efficacy of TAK-079 in Patients With Generalized Myasthenia Gravis. U.S.National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT03125577) Combination CAR-T Cell Therapy Targeting Hematological Malignancies | |||||
| REF 7 | ClinicalTrials.gov (NCT03638206) Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | |||||
| REF 8 | ClinicalTrials.gov (NCT03767751) A Feasibility and Safety Study of Dual Specificity CD38 and BCMA CAR-T Cell Immunotherapy for Relapsed or Refractory Multiple Myeloma | |||||
| REF 9 | ClinicalTrials.gov (NCT03271632) Multi-CAR T Cell Therapy in the Treatment of Multiple Myeloma | |||||
| REF 10 | ClinicalTrials.gov (NCT03754764) A Feasibility and Safety Study of CD38 CAR-T Cell Immunotherapy for Relapsed B-cell Acute Lymphoblastic Leukemia After CD19 CAR-T Adoptive Cellular Immunotherapy | |||||
| REF 11 | ClinicalTrials.gov (NCT03222674) Multi-CAR T Cell Therapy for Acute Myeloid Leukemia | |||||
| REF 12 | ClinicalTrials.gov (NCT03309111) Study of ISB 1342, a CD38/CD3 Bispecific Antibody, in Subjects With Previously Treated Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT01421186) A Phase I/IIa Study of Human Anti-CD38 Antibody MOR03087 (MOR202) in Relapsed/Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT01084252) Phase 1/2 Dose Escalation and Efficacy Study of Anti-CD38 Monoclonal Antibody in Patients With Selected CD38+ Hematological Malignancies. U.S. National Institutes of Health. | |||||
| REF 15 | ClinicalTrials.gov (NCT02998047) A Phase I Study of Lintuzumab-Ac225 in Patients With Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 16 | ClinicalTrials.gov (NCT03445663) Study Evaluating AMG 424 in Subjects With Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT03464916) Study to Evaluate the Safety and Efficacy of Anti-CD38 CAR-T in Relapsed or Refractory Multiple Myeloma Patients | |||||
| REF 18 | ClinicalTrials.gov (NCT03291444) CAR-T Cells Combined With Peptide Specific Dendritic Cell in Relapsed/Refractory Leukemia/MDS | |||||
| REF 19 | Clinical pipeline report, company report or official report of Sorrento Therapeutics. | |||||
| REF 20 | ClinicalTrials.gov (NCT04634435) A Phase 1 Study of Autologous Memory-like Natural Killer (NK) Cell Immunotherapy With BHV-1100 (Formerly KP1237) and IVIG Followed by Low Dose IL-2 as Early Post-Autologous Transplant Consolidation in Minimal Residual Disease Positive, Multiple Myeloma (MM) Patients in First or Second Remission. U.S.National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT04000282) First-in-human Single Agent Study of SAR442085 in Relapsed or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT04401020) First-in-human Single Agent Study of SAR442257 in RRMM and RR-NHL. U.S. National Institutes of Health. | |||||
| REF 23 | Clinical pipeline report, company report or official report of Sanofi | |||||
| REF 24 | ClinicalTrials.gov (NCT04017130) A Study of TAK-169 in Participants With Relapsed or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||||
| REF 25 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 26 | ClinicalTrials.gov (NCT03473496) CAR-T Cells Therapy in Relapsed/Refractory Multiple Myeloma | |||||
| REF 27 | Safety, tolerability, pharmacokinetics and pharmacodynamics of the anti-CD38 cytolytic antibody TAK-079 in healthy subjects. Br J Clin Pharmacol. 2020 Jul;86(7):1314-1325. | |||||
| REF 28 | Clinical pipeline report, company report or official report of Ichnos Sciences. | |||||
| REF 29 | Clinical pipeline report, company report or official report of MorphoSys. | |||||
| REF 30 | Clinical pipeline report, company report or official report of Sanofi-Aventis. | |||||
| REF 31 | Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020 Sep;19(9):589-608. | |||||
| REF 32 | Clinical pipeline report, company report or official report of Amgen. | |||||
| REF 33 | Clinical pipeline report, company report or official report of Sanofi. | |||||
| REF 34 | Clinical pipeline report, company report or official report of Molecular Templates. | |||||
| REF 35 | Clinical pipeline report, company report or official report of Takeda. | |||||
| REF 36 | ClinicalTrials.gov (NCT03473457) CAR-T Cells Therapy in Relapsed/Refractory Acute Myeloid Leukemia | |||||
| REF 37 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2766). | |||||
| REF 38 | Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38. Chem Biol. 2008 Oct 20;15(10):1068-78. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

