Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T10965
(Former ID: TTDC00197)
|
|||||
| Target Name |
P-selectin (SELP)
|
|||||
| Synonyms |
SELP; PADGEM; Leukocyte-endothelial cell adhesion molecule 3; LECAM3; Granule membrane protein 140; GMP-140; CD62P
Click to Show/Hide
|
|||||
| Gene Name |
SELP
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Circulatory system disease [ICD-11: BE2Z] | |||||
| 2 | Sickle-cell disorder [ICD-11: 3A51] | |||||
| Function |
Ca(2+)-dependent receptor for myeloid cells that binds to carbohydrates on neutrophils and monocytes. Mediates the interaction of activated endothelial cells or platelets with leukocytes. The ligand recognized is sialyl-Lewis X. Mediates rapid rolling of leukocyte rolling over vascular surfaces during the initial steps in inflammation through interaction with PSGL1.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MANCQIAILYQRFQRVVFGISQLLCFSALISELTNQKEVAAWTYHYSTKAYSWNISRKYC
QNRYTDLVAIQNKNEIDYLNKVLPYYSSYYWIGIRKNNKTWTWVGTKKALTNEAENWADN EPNNKRNNEDCVEIYIKSPSAPGKWNDEHCLKKKHALCYTASCQDMSCSKQGECLETIGN YTCSCYPGFYGPECEYVRECGELELPQHVLMNCSHPLGNFSFNSQCSFHCTDGYQVNGPS KLECLASGIWTNKPPQCLAAQCPPLKIPERGNMTCLHSAKAFQHQSSCSFSCEEGFALVG PEVVQCTASGVWTAPAPVCKAVQCQHLEAPSEGTMDCVHPLTAFAYGSSCKFECQPGYRV RGLDMLRCIDSGHWSAPLPTCEAISCEPLESPVHGSMDCSPSLRAFQYDTNCSFRCAEGF MLRGADIVRCDNLGQWTAPAPVCQALQCQDLPVPNEARVNCSHPFGAFRYQSVCSFTCNE GLLLVGASVLQCLATGNWNSVPPECQAIPCTPLLSPQNGTMTCVQPLGSSSYKSTCQFIC DEGYSLSGPERLDCTRSGRWTDSPPMCEAIKCPELFAPEQGSLDCSDTRGEFNVGSTCHF SCDNGFKLEGPNNVECTTSGRWSATPPTCKGIASLPTPGLQCPALTTPGQGTMYCRHHPG TFGFNTTCYFGCNAGFTLIGDSTLSCRPSGQWTAVTPACRAVKCSELHVNKPIAMNCSNL WGNFSYGSICSFHCLEGQLLNGSAQTACQENGHWSTTVPTCQAGPLTIQEALTYFGGAVA STIGLIMGGTLLALLRKRFRQKDDGKCPLNPHSHLGTYGVFTNAAFDPSP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A05438 ; BADD_A06761 | |||||
| HIT2.0 ID | T07NML | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Crizanlizumab | Drug Info | Approved | Vaso-occlusive crisis | [2] | |
| Clinical Trial Drug(s) | [+] 7 Clinical Trial Drugs | + | ||||
| 1 | GMI-1070 | Drug Info | Phase 3 | Asthma | [1], [3], [4] | |
| 2 | Inclacumab | Drug Info | Phase 3 | Sickle-cell disorder | [5] | |
| 3 | CY-1503 | Drug Info | Phase 2/3 | Hypertension | [6] | |
| 4 | BAICALEIN | Drug Info | Phase 2 | Influenza virus infection | [7] | |
| 5 | RPSGL-Ig | Drug Info | Phase 2 | Delayed graft function | [8] | |
| 6 | SelG1 | Drug Info | Phase 2 | Vaso-occlusive crisis | [9] | |
| 7 | PSI-697 | Drug Info | Phase 1 | Thrombosis | [10] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | CDP-850 | Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | [11] | |
| 2 | CY-1787 | Drug Info | Discontinued in Phase 1 | Allergy | [12] | |
| 3 | SMART anti-E/P selectin | Drug Info | Discontinued in Phase 1 | Asthma | [13] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 14 Inhibitor drugs | + | ||||
| 1 | Crizanlizumab | Drug Info | [14] | |||
| 2 | GMI-1070 | Drug Info | [1], [4], [15] | |||
| 3 | Inclacumab | Drug Info | [16] | |||
| 4 | BAICALEIN | Drug Info | [18] | |||
| 5 | RPSGL-Ig | Drug Info | [19], [8] | |||
| 6 | PSI-697 | Drug Info | [21] | |||
| 7 | PURPUROGALLIN | Drug Info | [18] | |||
| 8 | 1na | Drug Info | [25] | |||
| 9 | 2,3,4-trihydroxybenzoic acid | Drug Info | [18] | |||
| 10 | 2-Methyl-2,4-Pentanediol | Drug Info | [25] | |||
| 11 | Efomycine M | Drug Info | [26] | |||
| 12 | Fucose | Drug Info | [25] | |||
| 13 | GALLICACID | Drug Info | [18] | |||
| 14 | O-Sialic Acid | Drug Info | [27] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | CY-1503 | Drug Info | [17] | |||
| 2 | CY-1787 | Drug Info | [23] | |||
| 3 | SMART anti-E/P selectin | Drug Info | [24] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
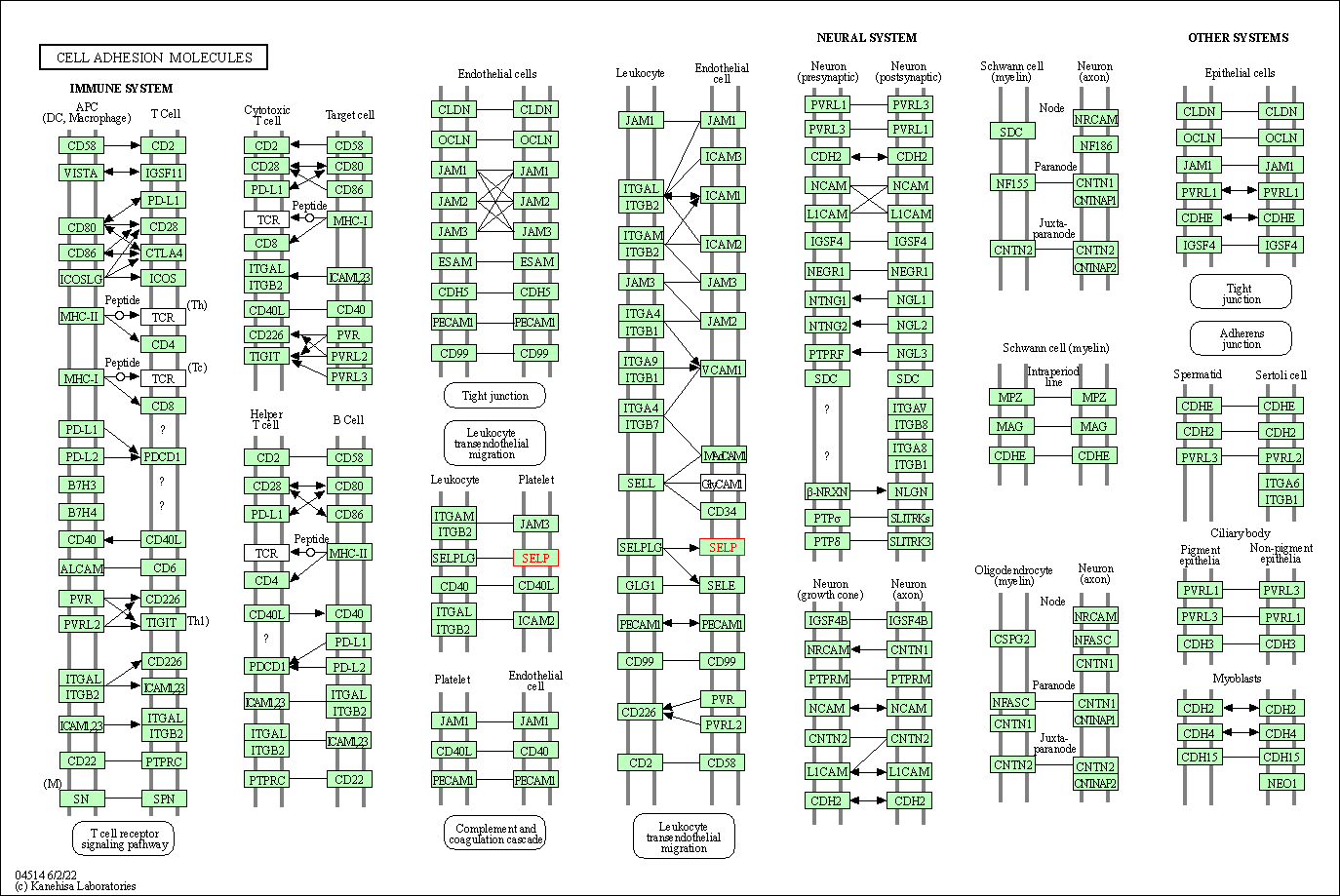
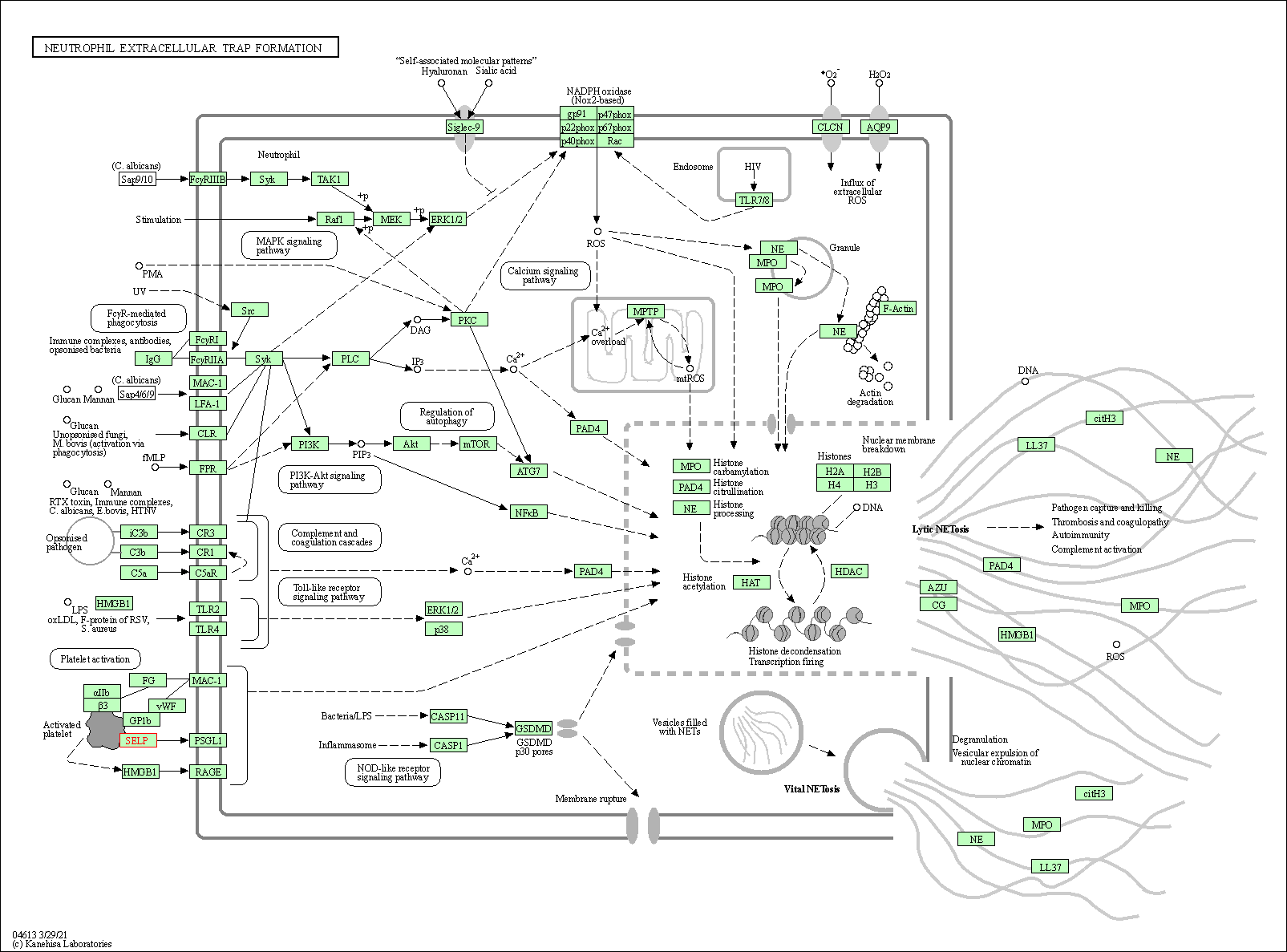
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cell adhesion molecules | hsa04514 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Neutrophil extracellular trap formation | hsa04613 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 12 | Degree centrality | 1.29E-03 | Betweenness centrality | 7.91E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.05E-01 | Radiality | 1.36E+01 | Clustering coefficient | 1.36E-01 |
| Neighborhood connectivity | 1.48E+01 | Topological coefficient | 1.11E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Cell adhesion molecules (CAMs) | |||||
| 2 | Malaria | |||||
| 3 | Staphylococcus aureus infection | |||||
| PID Pathway | [+] 2 PID Pathways | + | ||||
| 1 | IL4-mediated signaling events | |||||
| 2 | amb2 Integrin signaling | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Platelet degranulation | |||||
| 2 | Cell surface interactions at the vascular wall | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | Human Complement System | |||||
| 2 | Spinal Cord Injury | |||||
| 3 | Cell surface interactions at the vascular wall | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Deal watch: Pfizer deal for selectin inhibitor highlights potential of glycomimetic drugs. Nat Rev Drug Discov. 2011 Dec 1;10(12):890. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8307). | |||||
| REF 4 | GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 2010 Sep 9;116(10):1779-86. | |||||
| REF 5 | ClinicalTrials.gov (NCT05348915) An Open-label Extension Study to Evaluate the Long-term Safety of Inclacumab Administered to Participants With Sickle Cell Disease Who Have Participated in an Inclacumab Clinical Trial. U.S.National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT00226369) Cylexin for Reduction of Reperfusion Injury in Infant Heart Surgery. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT03830684) A Randomized, Double-blind, Placebo-controlled, Multicenter and Phase IIa Clinical Trial for the Effectiveness and Safety of Baicalein Tablets in the Treatment of Improve Other Aspects of Healthy Adult With Influenza Fever. U.S. National Institutes of Health. | |||||
| REF 8 | New developments in immunosuppressive therapy for heart transplantation. Expert Opin Emerg Drugs. 2009 Mar;14(1):1-21. | |||||
| REF 9 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 10 | ClinicalTrials.gov (NCT00367692) Study Evaluating PSI-697 in Patients With Scleritis. U.S. National Institutes of Health. | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007242) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003725) | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008129) | |||||
| REF 14 | Crizanlizumab: First Approval. Drugs. 2020 Jan;80(1):79-84. | |||||
| REF 15 | GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 2010 September 9; 116(10): 1779-1786. | |||||
| REF 16 | Analytical comparability demonstrated for an IgG4 molecule, inclacumab, following transfer of manufacturing responsibility from Roche to Global Blood Therapeutics. Expert Opin Biol Ther. 2022 Nov;22(11):1417-1428. | |||||
| REF 17 | Adjunctive selectin blockade successfully reduces infarct size beyond thrombolysis in the electrolytic canine coronary artery model. Circulation. 1995 Aug 1;92(3):492-9. | |||||
| REF 18 | Rational design of novel, potent small molecule pan-selectin antagonists. J Med Chem. 2007 Mar 22;50(6):1101-15. | |||||
| REF 19 | rPSGL-1-Ig, a recombinant PSGL-1-Ig fusion protein, ameliorates LPS-induced acute lung injury in mice by inhibiting neutrophil migration. Cell Mol Biol (Noisy-le-grand). 2015 Feb 28;61(1):1-6. | |||||
| REF 20 | Clinical pipeline report, company report or official report of Selexys Pharmaceuticals (2011). | |||||
| REF 21 | Effect of PSI-697, a novel P-selectin inhibitor, on platelet-monocyte aggregate formation in humans. J Am Heart Assoc. 2013 Jan 28;2(1):e006007. | |||||
| REF 22 | WO patent application no. 2001,0276,21, Competitive inhibition elisa for antibody detection. | |||||
| REF 23 | Administration of an antibody to E-selectin in patients with septic shock. Crit Care Med. 1996 Feb;24(2):229-33. | |||||
| REF 24 | HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ Res. 2002 Nov 15;91(10):907-14. | |||||
| REF 25 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 26 | Interfering with leukocyte rolling--a promising therapeutic approach in inflammatory skin disorders Trends Pharmacol Sci. 2003 Feb;24(2):49-52. | |||||
| REF 27 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

