Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T28722
(Former ID: TTDI01774)
|
|||||
| Target Name |
GABA(A) receptor gamma-3 (GABRG3)
|
|||||
| Synonyms |
GABRG3
Click to Show/Hide
|
|||||
| Gene Name |
GABRG3
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 9 Target-related Diseases | + | ||||
| 1 | Anxiety disorder [ICD-11: 6B00-6B0Z] | |||||
| 2 | Corneal disease [ICD-11: 9A76-9A78] | |||||
| 3 | Depression [ICD-11: 6A70-6A7Z] | |||||
| 4 | Epilepsy/seizure [ICD-11: 8A61-8A6Z] | |||||
| 5 | Insomnia [ICD-11: 7A00-7A0Z] | |||||
| 6 | Intentional self-harm [ICD-11: PC91] | |||||
| 7 | Labour/delivery anaesthesia complication [ICD-11: JB0C] | |||||
| 8 | Mental/behavioural/neurodevelopmental disorder [ICD-11: 6E20-6E8Z] | |||||
| 9 | Mood/affect symptom [ICD-11: MB24] | |||||
| Function |
Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functionsas receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand- gated chloride channel.
Click to Show/Hide
|
|||||
| BioChemical Class |
Ligand-gated ion channel
|
|||||
| UniProt ID | ||||||
| Sequence |
MAPKLLLLLCLFSGLHARSRKVEEDEYEDSSSNQKWVLAPKSQDTDVTLILNKLLREYDK
KLRPDIGIKPTVIDVDIYVNSIGPVSSINMEYQIDIFFAQTWTDSRLRFNSTMKILTLNS NMVGLIWIPDTIFRNSKTAEAHWITTPNQLLRIWNDGKILYTLRLTINAECQLQLHNFPM DEHSCPLIFSSYGYPKEEMIYRWRKNSVEAADQKSWRLYQFDFMGLRNTTEIVTTSAGDY VVMTIYFELSRRMGYFTIQTYIPCILTVVLSWVSFWIKKDATPARTALGITTVLTMTTLS TIARKSLPRVSYVTAMDLFVTVCFLFVFAALMEYATLNYYSSCRKPTTTKKTTSLLHPDS SRWIPERISLQAPSNYSLLDMRPPPTAMITLNNSVYWQEFEDTCVYECLDGKDCQSFFCC YEECKSGSWRKGRIHIDILELDSYSRVFFPTSFLLFNLVYWVGYLYL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 13 Approved Drugs | + | ||||
| 1 | Allopregnanolone | Drug Info | Approved | Postpartum depression | [2], [3] | |
| 2 | Bentazepam | Drug Info | Approved | Anxiety disorder | [4] | |
| 3 | Clobazam - Lundbeck | Drug Info | Approved | Anxiety disorder | [5], [6] | |
| 4 | Etomidate | Drug Info | Approved | Anaesthesia | [7], [8] | |
| 5 | Flumazenil | Drug Info | Approved | Benzodiazepine overdose | [9], [10] | |
| 6 | Mebutamate | Drug Info | Approved | Anxiety disorder | [4] | |
| 7 | Metharbital | Drug Info | Approved | Epilepsy | [11], [12] | |
| 8 | Remimazolam | Drug Info | Approved | Procedural sedation | [13] | |
| 9 | Stiripentol | Drug Info | Approved | Dravet syndrome | [14] | |
| 10 | Talbutal | Drug Info | Approved | Irritability | [15] | |
| 11 | Thiamylal | Drug Info | Approved | Anaesthesia | [16], [17] | |
| 12 | Zolpidem | Drug Info | Approved | Insomnia | [4], [18] | |
| 13 | Zuranolone | Drug Info | Approved | Postpartum depression | [19] | |
| Clinical Trial Drug(s) | [+] 10 Clinical Trial Drugs | + | ||||
| 1 | Arbaclofen placarbil | Drug Info | Phase 3 | Fragile X syndrome | [20] | |
| 2 | Clomethiazole | Drug Info | Phase 3 | Stroke | [21] | |
| 3 | Pagoclone | Drug Info | Phase 2/3 | Anxiety disorder | [22] | |
| 4 | Etazolate | Drug Info | Phase 2 | Neurodegenerative disorder | [23], [24] | |
| 5 | EVT-201 | Drug Info | Phase 2 | Insomnia | [25] | |
| 6 | SARIPIDEM | Drug Info | Phase 2 | Anxiety disorder | [26] | |
| 7 | T-2007 | Drug Info | Phase 2 | Epilepsy | [27] | |
| 8 | AZD-3043 | Drug Info | Phase 1 | Anaesthesia | [28] | |
| 9 | NSD-788 | Drug Info | Phase 1 | Anxiety disorder | [29] | |
| 10 | Org-25435 | Drug Info | Phase 1 | Epilepsy | [30] | |
| Discontinued Drug(s) | [+] 21 Discontinued Drugs | + | ||||
| 1 | Suriclone | Drug Info | Discontinued in Preregistration | Anxiety disorder | [31] | |
| 2 | Ocinaplon | Drug Info | Discontinued in Phase 3 | Generalized anxiety disorder | [32], [33] | |
| 3 | Pazinaclone | Drug Info | Discontinued in Phase 3 | Anxiety disorder | [34] | |
| 4 | Y-23684 | Drug Info | Discontinued in Phase 3 | Anxiety disorder | [35] | |
| 5 | LORECLEZOLE | Drug Info | Discontinued in Phase 2 | Epileptic seizures | [36], [37] | |
| 6 | NGD 91-3 | Drug Info | Discontinued in Phase 2 | Anxiety disorder | [38] | |
| 7 | RESEQUINIL | Drug Info | Discontinued in Phase 2 | Epilepsy | [39] | |
| 8 | RO-48-6791 | Drug Info | Discontinued in Phase 2 | Anxiety disorder | [40] | |
| 9 | SL-65.1498 | Drug Info | Discontinued in Phase 2 | Anxiety disorder | [41] | |
| 10 | Suritozole | Drug Info | Discontinued in Phase 2 | Major depressive disorder | [42] | |
| 11 | CCD-3693 | Drug Info | Discontinued in Phase 1 | Anxiety disorder | [43] | |
| 12 | CTP-354 | Drug Info | Discontinued in Phase 1 | Pain | [44] | |
| 13 | Org-21465 | Drug Info | Discontinued in Phase 1 | Anaesthesia | [45] | |
| 14 | RO-48-8684 | Drug Info | Discontinued in Phase 1 | Anxiety disorder | [46] | |
| 15 | Co-152791 | Drug Info | Terminated | Epilepsy | [48] | |
| 16 | Girisopam | Drug Info | Terminated | Anxiety disorder | [49] | |
| 17 | NSD-721 | Drug Info | Terminated | Anxiety disorder | [50] | |
| 18 | Ro-19-8022 | Drug Info | Terminated | Anxiety disorder | [51] | |
| 19 | RU-33965 | Drug Info | Terminated | Alzheimer disease | [52] | |
| 20 | ZK-91296 | Drug Info | Terminated | Alzheimer disease | [53] | |
| 21 | ZK-93426 | Drug Info | Terminated | Alzheimer disease | [54], [55] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | RWJ-51204 | Drug Info | Preclinical | Anxiety disorder | [47] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Modulator | [+] 48 Modulator drugs | + | ||||
| 1 | Allopregnanolone | Drug Info | [3], [56] | |||
| 2 | Bentazepam | Drug Info | [1], [4] | |||
| 3 | Clobazam - Lundbeck | Drug Info | [57] | |||
| 4 | Etomidate | Drug Info | [57] | |||
| 5 | Mebutamate | Drug Info | [57] | |||
| 6 | Metharbital | Drug Info | [57] | |||
| 7 | Stiripentol | Drug Info | [4], [59] | |||
| 8 | Talbutal | Drug Info | [57] | |||
| 9 | Thiamylal | Drug Info | [57] | |||
| 10 | Zuranolone | Drug Info | [19] | |||
| 11 | Clomethiazole | Drug Info | [60] | |||
| 12 | Pagoclone | Drug Info | [61] | |||
| 13 | Etazolate | Drug Info | [24] | |||
| 14 | EVT-201 | Drug Info | [58] | |||
| 15 | SARIPIDEM | Drug Info | [26] | |||
| 16 | AZD-3043 | Drug Info | [62] | |||
| 17 | NSD-788 | Drug Info | [58] | |||
| 18 | Org-25435 | Drug Info | [63] | |||
| 19 | Suriclone | Drug Info | [64] | |||
| 20 | Ocinaplon | Drug Info | [65] | |||
| 21 | Pazinaclone | Drug Info | [66] | |||
| 22 | Y-23684 | Drug Info | [67] | |||
| 23 | LORECLEZOLE | Drug Info | [68] | |||
| 24 | RO-48-6791 | Drug Info | [71] | |||
| 25 | Suritozole | Drug Info | [73] | |||
| 26 | CCD-3693 | Drug Info | [74] | |||
| 27 | CTP-354 | Drug Info | [75] | |||
| 28 | Org-21465 | Drug Info | [76] | |||
| 29 | RO-48-8684 | Drug Info | [77] | |||
| 30 | RWJ-51204 | Drug Info | [78] | |||
| 31 | Co-152791 | Drug Info | [79] | |||
| 32 | Girisopam | Drug Info | [80] | |||
| 33 | NSD-721 | Drug Info | [58] | |||
| 34 | Ro-19-8022 | Drug Info | [81] | |||
| 35 | RU-33965 | Drug Info | [82], [83], [84] | |||
| 36 | ZK-91296 | Drug Info | [85] | |||
| 37 | ZK-93426 | Drug Info | [86] | |||
| 38 | AA-29504 | Drug Info | [58] | |||
| 39 | C-21191 | Drug Info | [58] | |||
| 40 | CP-409092 | Drug Info | [87] | |||
| 41 | DOV-51892 | Drug Info | [58] | |||
| 42 | GIDAZEPAM | Drug Info | [88] | |||
| 43 | HZ-166 | Drug Info | [58] | |||
| 44 | NGD 96-3 | Drug Info | [58] | |||
| 45 | PNU 101017 | Drug Info | [89] | |||
| 46 | Ro-15-3505 | Drug Info | [90] | |||
| 47 | UC-2024 | Drug Info | [58] | |||
| 48 | UC-2029 | Drug Info | [58] | |||
| Modulator (allosteric modulator) | [+] 10 Modulator (allosteric modulator) drugs | + | ||||
| 1 | Flumazenil | Drug Info | [58] | |||
| 2 | Zolpidem | Drug Info | [58] | |||
| 3 | alpha3IA | Drug Info | [58] | |||
| 4 | alpha5IA | Drug Info | [58] | |||
| 5 | DMCM | Drug Info | [58] | |||
| 6 | tetrahydrodeoxycorticosterone | Drug Info | [58] | |||
| 7 | TP003 | Drug Info | [58] | |||
| 8 | [18F]fluoroethylflumazenil | Drug Info | [58] | |||
| 9 | [3H]CGS8216 | Drug Info | [58] | |||
| 10 | [3H]Ro154513 | Drug Info | [58] | |||
| Agonist | [+] 9 Agonist drugs | + | ||||
| 1 | Remimazolam | Drug Info | [13] | |||
| 2 | Arbaclofen placarbil | Drug Info | [3] | |||
| 3 | T-2007 | Drug Info | [58] | |||
| 4 | NGD 91-3 | Drug Info | [69] | |||
| 5 | RESEQUINIL | Drug Info | [70] | |||
| 6 | SL-65.1498 | Drug Info | [72] | |||
| 7 | isonipecotic acid | Drug Info | [58] | |||
| 8 | JM-1232(-) | Drug Info | [58] | |||
| 9 | piperidine-4-sulphonic acid | Drug Info | [58] | |||
| Blocker (channel blocker) | [+] 2 Blocker (channel blocker) drugs | + | ||||
| 1 | TBPS | Drug Info | [58] | |||
| 2 | [35S]TBPS | Drug Info | [58] | |||
| Antagonist | [+] 1 Antagonist drugs | + | ||||
| 1 | UC-1011 | Drug Info | [58] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Pathway Affiliation
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
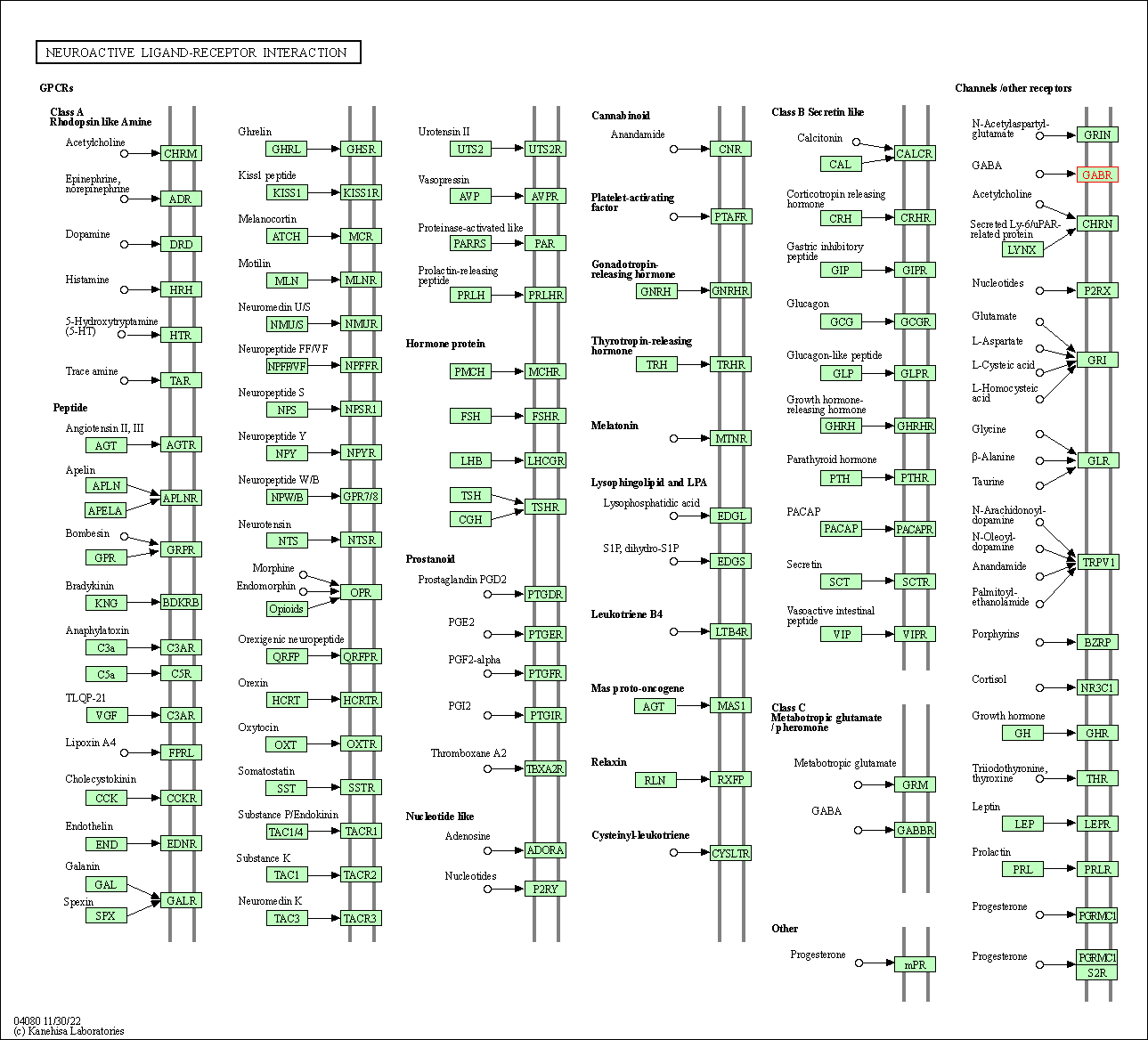
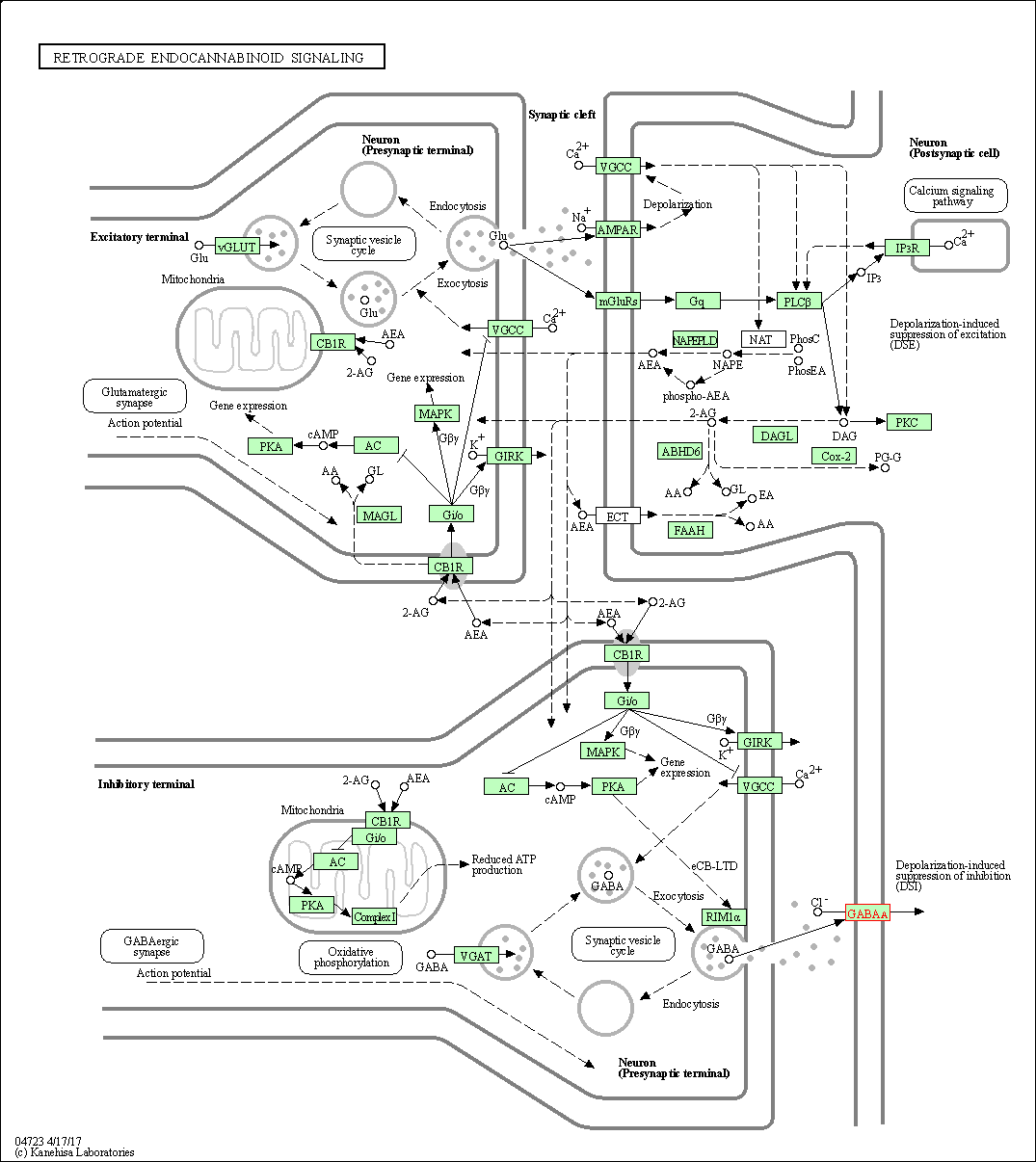
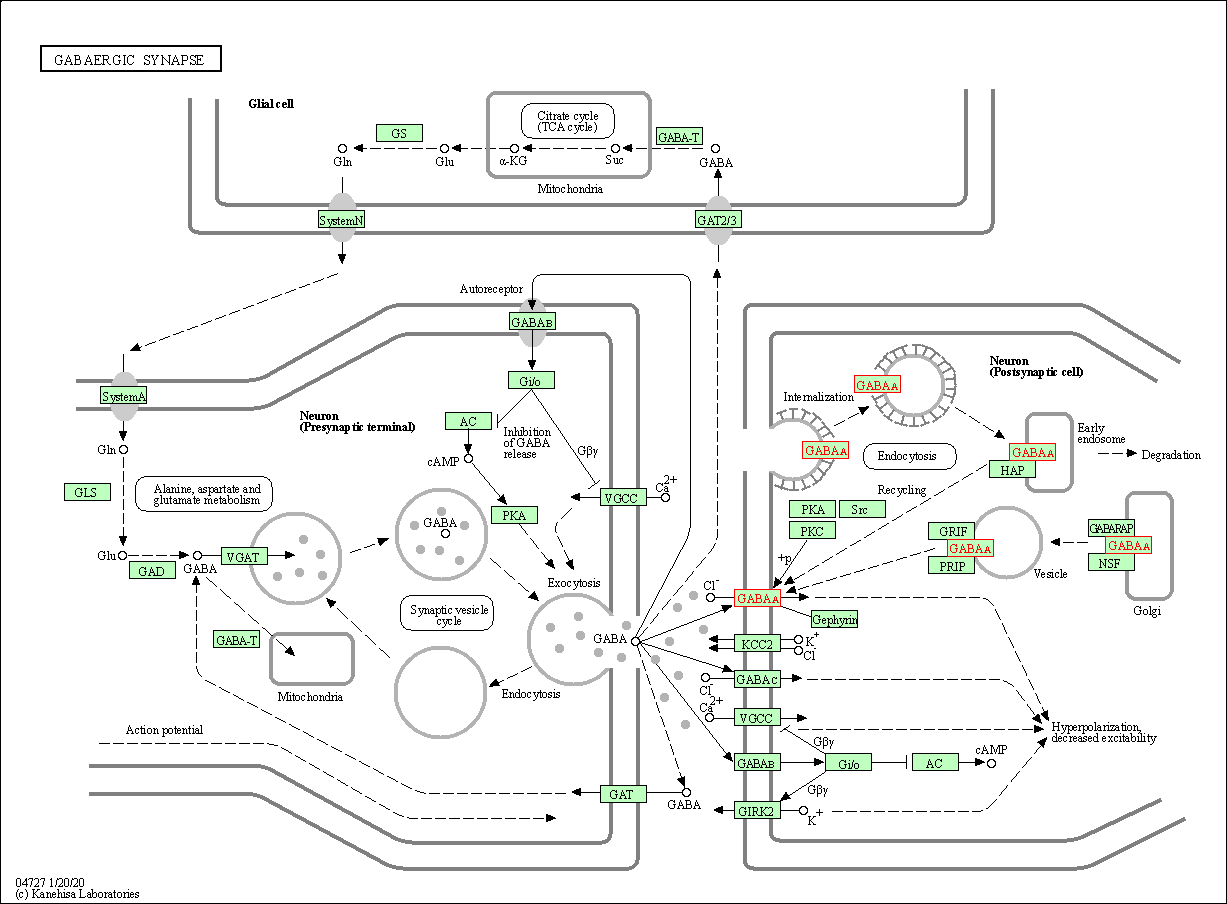
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Retrograde endocannabinoid signaling | hsa04723 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| GABAergic synapse | hsa04727 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Neuroactive ligand-receptor interaction | |||||
| 2 | Retrograde endocannabinoid signaling | |||||
| 3 | GABAergic synapse | |||||
| 4 | Morphine addiction | |||||
| 5 | Nicotine addiction | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Ligand-gated ion channel transport | |||||
| 2 | GABA A receptor activation | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | SIDS Susceptibility Pathways | |||||
| 2 | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| 3 | Iron uptake and transport | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Prodynorphin gene deletion increased anxiety-like behaviours, impaired the anxiolytic effect of bromazepam and altered GABAA receptor subunits gene expression in the amygdala. J Psychopharmacol. 2011Jan;25(1):87-96. | |||||
| REF 2 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7149). | |||||
| REF 6 | Drug information of Clobazam, 2008. eduDrugs. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5463). | |||||
| REF 8 | Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12(28):3665-79. | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4192). | |||||
| REF 10 | ClinicalTrials.gov (NCT00997087) A Randomized, Double-Blind, Placebo-Controlled Trial of Flumazenil for the Treatment of Obsessive Compulsive Disorder. U.S. National Institutes of Health. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7230). | |||||
| REF 12 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 008322. | |||||
| REF 13 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 14 | The effects of stiripentol on GABA(A) receptors.Epilepsia. 2011 Apr;52 Suppl 2:76-8. | |||||
| REF 15 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 009410. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7305). | |||||
| REF 17 | Drug information of Thiamylal, 2008. eduDrugs. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4348). | |||||
| REF 19 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 217369 | |||||
| REF 20 | ClinicalTrials.gov (NCT01325220) Efficacy and Safety Study of STX209 (Arbaclofen) for the Treatment of Social Withdrawal in Children With Fragile X Syndrome. U.S. National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT02374567) Pharmacovigilance in Gerontopsychiatric Patients. U.S. National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT00830154) A Study to Assess the Efficacy and Safety of Pagoclone for Adults With Stuttering. U.S. National Institutes of Health. | |||||
| REF 23 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7336). | |||||
| REF 24 | Etazolate, a neuroprotective drug linking GABA(A) receptor pharmacology to amyloid precursor protein processing. J Neurochem. 2008 Jul;106(1):392-404. | |||||
| REF 25 | ClinicalTrials.gov (NCT00380003) Efficacy Study of EVT 201 to Treat Insomnia. U.S. National Institutes of Health. | |||||
| REF 26 | Behavioural effects of novel benzodiazepine (omega) receptor agonists and partial agonists: increases in punished responding and antagonism of the pentylenetetrazole cue. Behav Pharmacol. 1995 Mar;6(2):116-126. | |||||
| REF 27 | ClinicalTrials.gov (NCT00939653) T2007-002 Clofarabine, Etoposide, Cyclophosphamide in Relapsed Acute Myelogenous Leukemia (AML). U.S. National Institutes of Health. | |||||
| REF 28 | ClinicalTrials.gov (NCT01086813) Phase I, Single Centre, Study to Assess Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AZD3043. U.S. National Institutes of Health. | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028006) | |||||
| REF 30 | ClinicalTrials.gov (NCT01062867) First Administration to Man Of Org 25435 a New Intravenous Anesthetic. U.S. National Institutes of Health. | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000574) | |||||
| REF 32 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4277). | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001407) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000717) | |||||
| REF 35 | The pharmacological properties of Y-23684, a benzodiazepine receptor partial agonist.. Br J Pharmacol. 1994 April; 111(4): 1170-1178. | |||||
| REF 36 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5466). | |||||
| REF 37 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007906) | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011480) | |||||
| REF 39 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010724) | |||||
| REF 40 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007556) | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014726) | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001322) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006723) | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038187) | |||||
| REF 45 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006459) | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007572) | |||||
| REF 47 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015754) | |||||
| REF 48 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011029) | |||||
| REF 49 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005437) | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010233) | |||||
| REF 51 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001584) | |||||
| REF 52 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001729) | |||||
| REF 53 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000552) | |||||
| REF 54 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4347). | |||||
| REF 55 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001474) | |||||
| REF 56 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 57 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 58 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 415). | |||||
| REF 59 | Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006 Apr;47(4):704-16. | |||||
| REF 60 | Electrophysiological actions of gamma-aminobutyric acid and clomethiazole on recombinant GABA(A) receptors. Eur J Pharmacol. 2002 Oct 11;452(3):255-62. | |||||
| REF 61 | Evaluation of the abuse potential of pagoclone, a partial GABAA agonist. J Clin Psychopharmacol. 2006 Jun;26(3):268-73. | |||||
| REF 62 | AZD-3043: a novel, metabolically labile sedative-hypnotic agent with rapid and predictable emergence from hypnosis. Anesthesiology. 2012 Jun;116(6):1267-77. | |||||
| REF 63 | First administration to man of Org 25435, an intravenous anaesthetic: A Phase 1 Clinical Trial. BMC Anesthesiol. 2010 Jun 29;10:10. | |||||
| REF 64 | The effect of cyclopyrrolones on GABAA receptor function is different from that of benzodiazepines. Naunyn Schmiedebergs Arch Pharmacol. 1994 Sep;350(3):294-300. | |||||
| REF 65 | Discriminative stimulus properties of GABAA receptor positive allosteric modulators TPA023, ocinaplon and NG2-73 in rats trained to discriminate chlordiazepoxide or zolpidem. Eur J Pharmacol. 2011 Oct 1;668(1-2):190-3. | |||||
| REF 66 | The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005 May;14(5):601-18. | |||||
| REF 67 | The pharmacological properties of Y-23684, a benzodiazepine receptor partial agonist. Br J Pharmacol. 1994 Apr;111(4):1170-8. | |||||
| REF 68 | Direct activation of GABAA receptors by loreclezole, an anticonvulsant drug with selectivity for the beta-subunit. Neuropharmacology. 1996;35(12):1753-60. | |||||
| REF 69 | Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site. Curr Drug Targets CNS Neurol Disord. 2003 Aug;2(4):213-32. | |||||
| REF 70 | WO patent application no. 2010,0024,51, Naphthyridin derivatives. | |||||
| REF 71 | Integrated pharmacokinetics and pharmacodynamics of Ro 48-6791, a new benzodiazepine, in comparison with midazolam during first administration to healthy male subjects. Br J Clin Pharmacol. 1997 Nov;44(5):477-86. | |||||
| REF 72 | WO patent application no. 2005,0749,31, Pharmaceutical combinations comprising (s) -pantoprazole. | |||||
| REF 73 | Chronic postinjury administration of MDL 26,479 (Suritozole), a negative modulator at the GABAA receptor, and cognitive impairment in rats following traumatic brain injury. J Neurosurg. 1995 Nov;83(5):878-83. | |||||
| REF 74 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 75 | Clinical pipeline report, company report or official report of Concert Pharmaceuticals. | |||||
| REF 76 | Computer-controlled infusion of ORG 21465, a water soluble steroid i.v. anaesthetic agent, into human volunteers. Br J Anaesth. 1997 Oct;79(4):433-9. | |||||
| REF 77 | Integrated pharmacokinetics and pharmacodynamics of Ro 48-8684, a new benzodiazepine, in comparison with midazolam during first administration to healthy male subjects. Br J Clin Pharmacol. 1997 Nov;44(5):487-93. | |||||
| REF 78 | 5-ethoxymethyl-7-fluoro-3-oxo-1,2,3,5-tetrahydrobenzo[4,5]imidazo[1,2a]pyridine-4-N-(2-fluorophenyl)carboxamide (RWJ-51204), a new nonbenzodiazepine anxiolytic. J Pharmacol Exp Ther. 2002 Nov;303(2):777-90. | |||||
| REF 79 | Substituted 3beta-phenylethynyl derivatives of 3alpha-hydroxy-5alpha-pregnan-20-one: remarkably potent neuroactive steroid modulators of gamma-aminobutyric acidA receptors. J Pharmacol Exp Ther. 1998Oct;287(1):198-207. | |||||
| REF 80 | [(3)H]-girisopam, a novel selective benzodiazepine for the 2, 3-benzodiazepine binding site. Brain Res Brain Res Protoc. 1999 Jul;4(2):230-5. | |||||
| REF 81 | Partial agonist of benzodiazepine receptors Ro 19-8022 elicits withdrawal symptoms after short-term administration in immature rats. Physiol Res. 2012;61(3):319-23. | |||||
| REF 82 | Discriminative stimulus properties of RU 33965, a benzodiazepine receptor weak partial inverse agonist. Pharmacol Biochem Behav. 1992 Oct;43(2):583-8. | |||||
| REF 83 | The effects of RU 33965 and RU 34030, two new 3-cyclopropyl carbonyl imidazobenzodiazepines, on GABAA receptor-mediated synaptic transmission in ce... Gen Pharmacol. 1994 May;25(3):589-97. | |||||
| REF 84 | Effects of benzodiazepine receptor inverse agonists and nicotine on behavioral vigilance in senescent rats. J Gerontol A Biol Sci Med Sci. 1996 May;51(3):B225-31. | |||||
| REF 85 | Enhancement of gamma-aminobutyric acid binding by the anxiolytic beta-carbolines ZK 93423 and ZK 91296. J Neurochem. 1987 May;48(5):1355-8. | |||||
| REF 86 | Actions of the beta-carboline ZK 93426 in an animal test of anxiety and the holeboard: interactions with Ro 15-1788. J Neural Transm. 1986;65(2):103-14. | |||||
| REF 87 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
| REF 88 | Discriminative effects of phenazepam and gidazepam in rats: comparison with other GABA-related drugs. Pharmacol Biochem Behav. 1999 Oct;64(2):397-401. | |||||
| REF 89 | Neuroprotective effects of the GABA(A) receptor partial agonist U-101017 in 3-acetylpyridine-treated rats. Neurosci Lett. 1997 May 30;228(1):45-9. | |||||
| REF 90 | Molecular structure and stereoelectronic properties of sarmazenil--a weak inverse agonist at the omega modulatory sites (benzodiazepine receptors):... Bioorg Med Chem. 1998 Oct;6(10):1745-57. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

