Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T82577
(Former ID: TTDS00181)
|
|||||
| Target Name |
Angiotensin-converting enzyme (ACE)
|
|||||
| Synonyms |
Kininase II; Dipeptidyl carboxypeptidase I; DCP1; DCP; CD143 antigen; CD143; ACE
Click to Show/Hide
|
|||||
| Gene Name |
SLC33A1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Cardiovascular disease [ICD-11: BA00-BE2Z] | |||||
| 2 | Heart failure [ICD-11: BD10-BD1Z] | |||||
| 3 | Hypertension [ICD-11: BA00-BA04] | |||||
| Function |
Able to inactivate bradykinin, a potent vasodilator. Has also a glycosidase activity which releases GPI-anchored proteins from the membrane by cleaving the mannose linkage in the GPI moiety. Converts angiotensin I to angiotensin II by release of the terminal His-Leu, this results in an increase of the vasoconstrictor activity of angiotensin.
Click to Show/Hide
|
|||||
| BioChemical Class |
Glycosylase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.2.1.-
|
|||||
| Sequence |
MGAASGRRGPGLLLPLPLLLLLPPQPALALDPGLQPGNFSADEAGAQLFAQSYNSSAEQV
LFQSVAASWAHDTNITAENARRQEEAALLSQEFAEAWGQKAKELYEPIWQNFTDPQLRRI IGAVRTLGSANLPLAKRQQYNALLSNMSRIYSTAKVCLPNKTATCWSLDPDLTNILASSR SYAMLLFAWEGWHNAAGIPLKPLYEDFTALSNEAYKQDGFTDTGAYWRSWYNSPTFEDDL EHLYQQLEPLYLNLHAFVRRALHRRYGDRYINLRGPIPAHLLGDMWAQSWENIYDMVVPF PDKPNLDVTSTMLQQGWNATHMFRVAEEFFTSLELSPMPPEFWEGSMLEKPADGREVVCH ASAWDFYNRKDFRIKQCTRVTMDQLSTVHHEMGHIQYYLQYKDLPVSLRRGANPGFHEAI GDVLALSVSTPEHLHKIGLLDRVTNDTESDINYLLKMALEKIAFLPFGYLVDQWRWGVFS GRTPPSRYNFDWWYLRTKYQGICPPVTRNETHFDAGAKFHVPNVTPYIRYFVSFVLQFQF HEALCKEAGYEGPLHQCDIYRSTKAGAKLRKVLQAGSSRPWQEVLKDMVGLDALDAQPLL KYFQPVTQWLQEQNQQNGEVLGWPEYQWHPPLPDNYPEGIDLVTDEAEASKFVEEYDRTS QVVWNEYAEANWNYNTNITTETSKILLQKNMQIANHTLKYGTQARKFDVNQLQNTTIKRI IKKVQDLERAALPAQELEEYNKILLDMETTYSVATVCHPNGSCLQLEPDLTNVMATSRKY EDLLWAWEGWRDKAGRAILQFYPKYVELINQAARLNGYVDAGDSWRSMYETPSLEQDLER LFQELQPLYLNLHAYVRRALHRHYGAQHINLEGPIPAHLLGNMWAQTWSNIYDLVVPFPS APSMDTTEAMLKQGWTPRRMFKEADDFFTSLGLLPVPPEFWNKSMLEKPTDGREVVCHAS AWDFYNGKDFRIKQCTTVNLEDLVVAHHEMGHIQYFMQYKDLPVALREGANPGFHEAIGD VLALSVSTPKHLHSLNLLSSEGGSDEHDINFLMKMALDKIAFIPFSYLVDQWRWRVFDGS ITKENYNQEWWSLRLKYQGLCPPVPRTQGDFDPGAKFHIPSSVPYIRYFVSFIIQFQFHE ALCQAAGHTGPLHKCDIYQSKEAGQRLATAMKLGFSRPWPEAMQLITGQPNMSASAMLSY FKPLLDWLRTENELHGEKLGWPQYNWTPNSARSEGPLPDSGRVSFLGLDLDAQQARVGQW LLLFLGIALLVATLGLSQRLFSIRHRSLHRHSHGPQFGSEVELRHS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A00592 ; BADD_A02552 | |||||
| HIT2.0 ID | T28PHS | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 20 Approved Drugs | + | ||||
| 1 | Alacepril | Drug Info | Approved | Cardiovascular disease | [2] | |
| 2 | Benazepril | Drug Info | Approved | Hypertension | [3], [4] | |
| 3 | Captopril | Drug Info | Approved | Hypertension | [5], [6] | |
| 4 | Cilazapril | Drug Info | Approved | Congestive heart failure | [4], [7] | |
| 5 | Delapril | Drug Info | Approved | Cardiovascular disease | [4] | |
| 6 | Deserpidine | Drug Info | Approved | Hypertension | [8], [9] | |
| 7 | Enalapril | Drug Info | Approved | Hypertension | [10], [11] | |
| 8 | Enalaprilat | Drug Info | Approved | Hypertension | [4], [12] | |
| 9 | Fosinopril | Drug Info | Approved | Hypertension | [4], [13] | |
| 10 | Hydrochlorothiazide | Drug Info | Approved | Hypertension | [14], [15] | |
| 11 | Imidapril | Drug Info | Approved | Cardiovascular disease | [2] | |
| 12 | Lisinopril | Drug Info | Approved | Hypertension | [4], [16] | |
| 13 | Moexipril | Drug Info | Approved | Hypertension | [4], [17] | |
| 14 | Perindopril | Drug Info | Approved | Hypertension | [4], [18] | |
| 15 | Quinapril | Drug Info | Approved | Hypertension | [4], [19] | |
| 16 | Ramipril | Drug Info | Approved | Congestive heart failure | [20], [21] | |
| 17 | Rescinnamine | Drug Info | Approved | Hypertension | [22], [23] | |
| 18 | Spirapril | Drug Info | Approved | Hypertension | [4], [24] | |
| 19 | Temocapril hydrochloride | Drug Info | Approved | Cardiovascular disease | [2] | |
| 20 | Trandolapril | Drug Info | Approved | Hypertension | [11], [25] | |
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | CONTIGOSIDE B | Drug Info | Phase 2/3 | Thrombosis | [26] | |
| 2 | Gallopamil | Drug Info | Phase 2 | Asthma | [27] | |
| 3 | GW-796406 | Drug Info | Phase 1 | Hypotension | [28] | |
| Discontinued Drug(s) | [+] 12 Discontinued Drugs | + | ||||
| 1 | Ilepatril | Drug Info | Discontinued in Phase 2/3 | Diabetic nephropathy | [29] | |
| 2 | Ceronapril | Drug Info | Discontinued in Phase 2 | Major depressive disorder | [30] | |
| 3 | Idrapril | Drug Info | Discontinued in Phase 2 | Hypertension | [31] | |
| 4 | M-100240 | Drug Info | Discontinued in Phase 2 | Hypotension | [32] | |
| 5 | METIAPRIL | Drug Info | Discontinued in Phase 2 | Hypotension | [33] | |
| 6 | UTIBAPRIL | Drug Info | Discontinued in Phase 2 | Hypertension | [34] | |
| 7 | Zabiciprilat | Drug Info | Discontinued in Phase 2 | Hypertension | [35] | |
| 8 | BMS-182657 | Drug Info | Terminated | Cardiovascular disease | [36] | |
| 9 | CGS-30440 | Drug Info | Terminated | Hypertension | [37] | |
| 10 | DU-1777 | Drug Info | Terminated | Hypertension | [38] | |
| 11 | GW-660511 | Drug Info | Terminated | Hypertension | [39] | |
| 12 | Omapatrilat | Drug Info | Terminated | Hypertension | [40] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 15 Modulator drugs | + | ||||
| 1 | Alacepril | Drug Info | [4], [41] | |||
| 2 | Delapril | Drug Info | [4], [41], [46], [47] | |||
| 3 | Hydrochlorothiazide | Drug Info | [52] | |||
| 4 | Imidapril | Drug Info | [4], [41] | |||
| 5 | Temocapril hydrochloride | Drug Info | [4], [41] | |||
| 6 | GW-796406 | Drug Info | [61] | |||
| 7 | Ilepatril | Drug Info | [29] | |||
| 8 | M-100240 | Drug Info | [64], [65] | |||
| 9 | BMS-182657 | Drug Info | [36] | |||
| 10 | CGS-30440 | Drug Info | [37] | |||
| 11 | GW-660511 | Drug Info | [39] | |||
| 12 | Omapatrilat | Drug Info | [40] | |||
| 13 | BRL-36378 | Drug Info | [73] | |||
| 14 | moexiprilat | Drug Info | [78], [79] | |||
| 15 | RB-105 | Drug Info | [81], [82], [83] | |||
| Inhibitor | [+] 38 Inhibitor drugs | + | ||||
| 1 | Benazepril | Drug Info | [42], [43] | |||
| 2 | Captopril | Drug Info | [44] | |||
| 3 | Cilazapril | Drug Info | [45] | |||
| 4 | Deserpidine | Drug Info | [1], [48] | |||
| 5 | Enalapril | Drug Info | [49] | |||
| 6 | Enalaprilat | Drug Info | [50] | |||
| 7 | Fosinopril | Drug Info | [51] | |||
| 8 | Lisinopril | Drug Info | [53] | |||
| 9 | Moexipril | Drug Info | [54] | |||
| 10 | Perindopril | Drug Info | [55] | |||
| 11 | Quinapril | Drug Info | [56] | |||
| 12 | Ramipril | Drug Info | [43] | |||
| 13 | Rescinnamine | Drug Info | [57] | |||
| 14 | Spirapril | Drug Info | [58] | |||
| 15 | Trandolapril | Drug Info | [59] | |||
| 16 | CONTIGOSIDE B | Drug Info | [60] | |||
| 17 | Gallopamil | Drug Info | [61] | |||
| 18 | Ceronapril | Drug Info | [62] | |||
| 19 | Idrapril | Drug Info | [63] | |||
| 20 | METIAPRIL | Drug Info | [66] | |||
| 21 | UTIBAPRIL | Drug Info | [67] | |||
| 22 | Zabiciprilat | Drug Info | [68] | |||
| 23 | DU-1777 | Drug Info | [69] | |||
| 24 | SCH-54470 | Drug Info | [70] | |||
| 25 | SQ-26332 | Drug Info | [71] | |||
| 26 | 2-(Acetylamino)-2-Deoxy-a-D-Glucopyranose | Drug Info | [72] | |||
| 27 | Acetate Ion | Drug Info | [72] | |||
| 28 | ASTRAGALIN | Drug Info | [60] | |||
| 29 | BUTEIN | Drug Info | [74] | |||
| 30 | fasidotrilat | Drug Info | [75] | |||
| 31 | Kaempferol-3-O-(2''-O-galloyl)-glucoside | Drug Info | [60] | |||
| 32 | lisinopril-tryptophan | Drug Info | [76] | |||
| 33 | LY-292223 | Drug Info | [77] | |||
| 34 | N-Carboxymethyl-N-cyclopentyl-phthalamic acid | Drug Info | [80] | |||
| 35 | RIP | Drug Info | [84] | |||
| 36 | RJM-0035-K002 | Drug Info | [85] | |||
| 37 | RXP-407 | Drug Info | [86] | |||
| 38 | [Cyclopentyl-(2-nitro-benzoyl)-amino]-acetic acid | Drug Info | [80] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: L-glutamine | Ligand Info | |||||
| Structure Description | Crystal structure of the Angiotensin-1 converting enzyme N-domain in complex with amyloid-beta 10-16 | PDB:5AM9 | ||||
| Method | X-ray diffraction | Resolution | 1.80 Å | Mutation | Yes | [87] |
| PDB Sequence |
LDPGLQPGQF
10 SADEAGAQLF20 AQSYQSSAEQ30 VLFQSVAASW40 AHDTNITAEN50 ARRQEEAALL 60 SQEFAEAWGQ70 KAKELYEPIW80 QQFTDPQLRR90 IIGAVRTLGS100 ANLPLAKRQQ 110 YNALLSQMSR120 IYSTAKVCLT135 CWSLDPDLTN145 ILASSRSYAM155 LLFAWEGWHN 165 AAGIPLKPLY175 EDFTALSNEA185 YKQDGFTDTG195 AYWRSWYNSP205 TFEDDLEHLY 215 QQLEPLYLNL225 HAFVRRALHR235 RYGDRYINLR245 GPIPAHLLGD255 MWAQSWENIY 265 DMVVPFPDKP275 NLDVTSTMLQ285 QGWQATHMFR295 VAEEFFTSLE305 LSPMPPEFWE 315 GSMLEKPADG325 REVVCHASAW335 DFYNRKDFRI345 KQCTRVTMDQ355 LSTVHHEMGH 365 IQYYLQYKDL375 PVSLRRGANP385 GFHEAIGDVL395 ALSVSTPEHL405 HKIGLLDRVT 415 NDTESDINYL425 LKMALEKIAF435 LPFGYLVDQW445 RWGVFSGRTP455 PSRYNFDWWY 465 LRTKYQGICP475 PVTRNETHFD485 AGAKFHVPNV495 TPYIRYFVSF505 VLQFQFHEAL 515 CKEAGYEGPL525 HQCDIYRSTK535 AGAKLRKVLR545 AGSSRPWQEV555 LKDMVGLDAL 565 DAQPLLKYFQ575 LVTQWLQEQN585 QQNGEVLGWP595 EYQWHPPLPD605 NYP |
|||||
|
|
||||||
| Ligand Name: Boric acid | Ligand Info | |||||
| Structure Description | Crystal structure of human Angiotensin-1 converting enzyme C-domain in complex with Omapatrilat. | PDB:6H5W | ||||
| Method | X-ray diffraction | Resolution | 1.37 Å | Mutation | Yes | [88] |
| PDB Sequence |
DEAEASKFVE
49 EYDRTSQVVW59 NEYAGANWNY69 NTNITTETSK79 ILLQKNMQIA89 QHTLKYGTQA 99 RKFDVNQLQN109 TTIKRIIKKV119 QDLERAALPA129 QELEEYNKIL139 LDMETTYSVA 149 TVCHPQGSCL159 QLEPDLTNVM169 ATSRKYEDLL179 WAWEGWRDKA189 GRAILQFYPK 199 YVELINQAAR209 LNGYVDAGDS219 WRSMYETPSL229 EQDLERLFQE239 LQPLYLNLHA 249 YVRRALHRHY259 GAQHINLEGP269 IPAHLLGNMW279 AQTWSNIYDL289 VVPFPSAPSM 299 DTTEAMLKQG309 WTPRRMFKEA319 DDFFTSLGLL329 PVPPEFWQKS339 MLEKPTDGRE 349 VVCHASAWDF359 YNGKDFRIKQ369 CTTVNLEDLV379 VAHHEMGHIQ389 YFMQYKDLPV 399 ALREGANPGF409 HEAIGDVLAL419 SVSTPKHLHS429 LNLLSSEGGS439 DEHDINFLMK 449 MALDKIAFIP459 FSYLVDQWRW469 RVFDGSITKE479 NYNQEWWSLR489 LKYQGLPPVP 500 RTQGDFDPGA510 KFHIPSSVPY520 IRYFVSFIIQ530 FQFHEALCQA540 AGHTGPLHKC 550 DIYQSKEAGQ560 RLATAMKLGF570 SRPWPEAMQL580 ITGQPQMSAS590 AMLSYFKPLL 600 DWLRTENELH610 GEKLGWPQYN620 WTPNS
|
|||||
|
|
THR282
4.092
TYR287
2.743
PHE293
3.842
SER298
3.110
VAL379
3.289
VAL380
4.027
HIS383
3.273
ASP415
2.713
PRO424
2.729
LEU427
4.643
LEU433
3.028
GLU436
2.666
ASP440
3.045
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
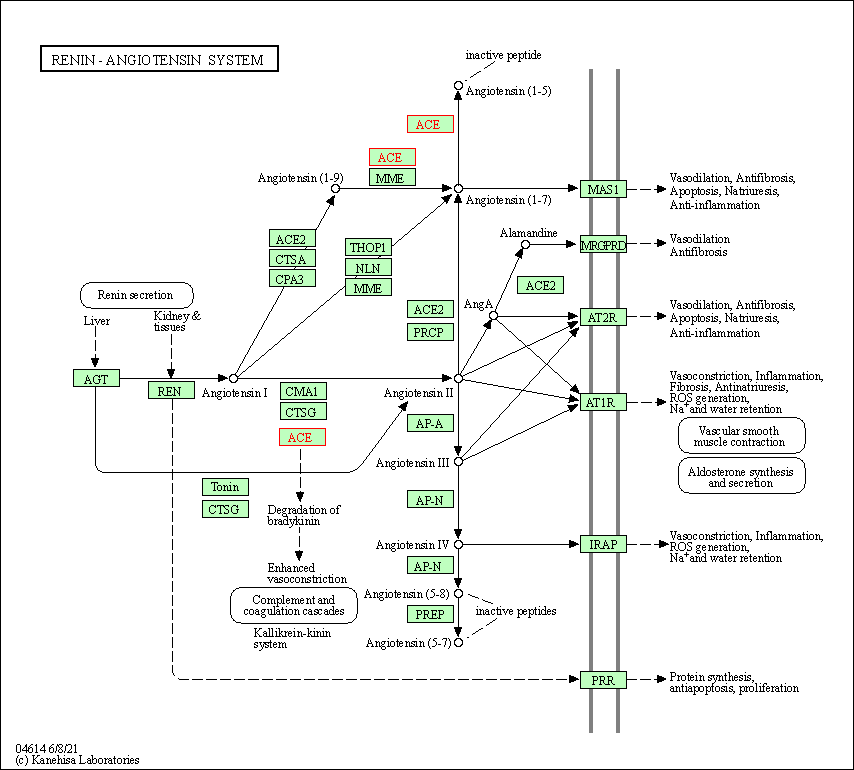
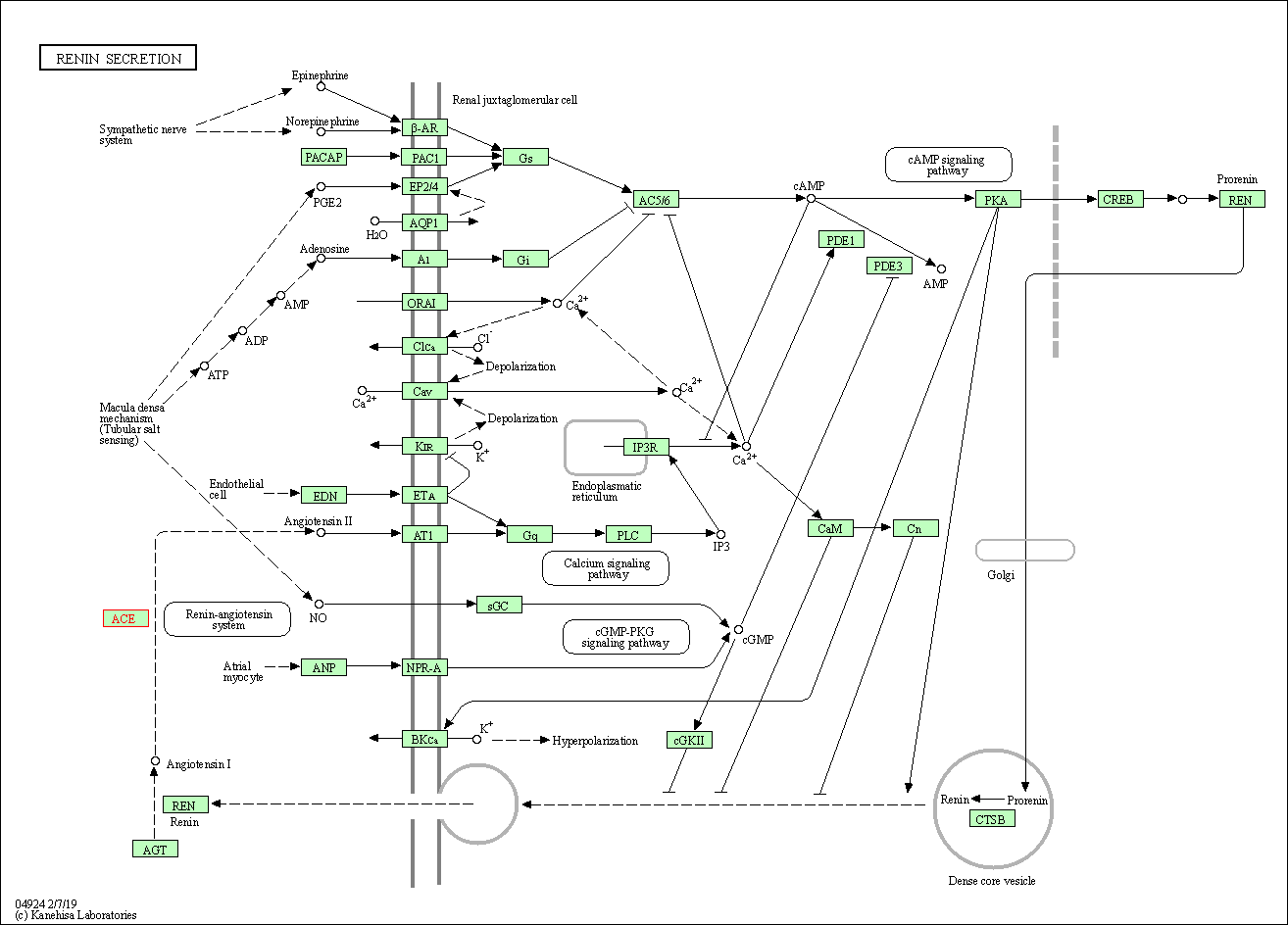
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Renin-angiotensin system | hsa04614 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Renin secretion | hsa04924 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 4 | Degree centrality | 4.30E-04 | Betweenness centrality | 1.59E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.11E-01 | Radiality | 1.37E+01 | Clustering coefficient | 1.67E-01 |
| Neighborhood connectivity | 4.15E+01 | Topological coefficient | 2.65E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Renin-angiotensin system | |||||
| 2 | Chagas disease (American trypanosomiasis) | |||||
| 3 | Hypertrophic cardiomyopathy (HCM) | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Angiotensin Metabolism | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Metabolism of Angiotensinogen to Angiotensins | |||||
| WikiPathways | [+] 2 WikiPathways | + | ||||
| 1 | ACE Inhibitor Pathway | |||||
| 2 | Metabolism of Angiotensinogen to Angiotensins | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Angiotensin II-induced modulation of endothelium-dependent relaxation in rabbit mesenteric resistance arteries. J Physiol. 2003 May 1;548(Pt 3):893-906. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6374). | |||||
| REF 4 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5158). | |||||
| REF 6 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 074322. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6459). | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7064). | |||||
| REF 9 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 010796. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6322). | |||||
| REF 11 | New antiarrhythmic agents for atrial fibrillation and atrial flutter. Expert Opin Emerg Drugs. 2005 May;10(2):311-22. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6332). | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6456). | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4836). | |||||
| REF 15 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040412. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6360). | |||||
| REF 17 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6571). | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6367). | |||||
| REF 19 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6350). | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6339). | |||||
| REF 21 | Emerging drugs in peripheral arterial disease. Expert Opin Emerg Drugs. 2006 Mar;11(1):75-90. | |||||
| REF 22 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7098). | |||||
| REF 23 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 010686. | |||||
| REF 24 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6575). | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6453). | |||||
| REF 26 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 27 | ClinicalTrials.gov (NCT00896428) Effects of Gallopamil in Severe Asthma (REMODEL'ASTHME) in University Hospital, Bordeaux. | |||||
| REF 28 | Mechanism of vasopeptidase inhibitor-induced plasma extravasation: comparison of omapatrilat and the novel neutral endopeptidase 24.11/angiotensin-... J Pharmacol Exp Ther. 2005 Dec;315(3):1306-13. | |||||
| REF 29 | Ilepatril (AVE-7688), a vasopeptidase inhibitor for the treatment of hypertension. Curr Opin Investig Drugs. 2008 Mar;9(3):301-9. | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000355) | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002045) | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002832) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005081) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000394) | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000139) | |||||
| REF 36 | Cardiovascular effects of the novel dual inhibitor of neutral endopeptidase and angiotensin-converting enzyme BMS-182657 in experimental hypertension and heart failure. J Pharmacol Exp Ther. 1995 Nov;275(2):745-52. | |||||
| REF 37 | Antihypertensive and natriuretic effects of CGS 30440, a dual inhibitor of angiotensin-converting enzyme and neutral endopeptidase 24.11. J Pharmacol Exp Ther. 1998 Mar;284(3):974-82. | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002041) | |||||
| REF 39 | The effects of Z13752A, a combined ACE/NEP inhibitor, on responses to coronary artery occlusion; a primary protective role for bradykinin. Br J Pharmacol. 2000 Feb;129(4):671-80. | |||||
| REF 40 | Omapatrilat, a dual angiotensin-converting enzyme and neutral endopeptidase inhibitor, prevents fatty streak deposit in apolipoprotein E-deficient mice. Atherosclerosis. 2001 Apr;155(2):291-5. | |||||
| REF 41 | Natural products in drug discovery and development. J Nat Prod. 1997 Jan;60(1):52-60. | |||||
| REF 42 | Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers. J Vet Intern Med. 2009 Sep-Oct;23(5):977-83. | |||||
| REF 43 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | |||||
| REF 44 | Using ACE inhibitors appropriately. Am Fam Physician. 2002 Aug 1;66(3):461-8. | |||||
| REF 45 | Triple pharmacological blockade of the renin-angiotensin-aldosterone system in nondiabetic CKD: an open-label crossover randomized controlled trial. Am J Kidney Dis. 2008 Sep;52(3):486-93. | |||||
| REF 46 | The influence of natural products upon drug discovery. Nat Prod Rep. 2000 Jun;17(3):215-34. | |||||
| REF 47 | Drug discovery from natural sources. AAPS J. 2006 Apr 14;8(2):E239-53. | |||||
| REF 48 | Drug interaction exposures in an ambulatory Medicaid population. Am J Hosp Pharm. 1979 Jul;36(7):923-7. | |||||
| REF 49 | Determination of bezafibrate, methotrexate, cyclophosphamide, orlistat and enalapril in waste and surface waters using on-line solid-phase extracti... J Environ Monit. 2009 Apr;11(4):830-8. | |||||
| REF 50 | Analysis of Vancomycin in the Hindlimb Vascular Bed of the Rat. Am J Ther. 1996 Oct;3(10):681-687. | |||||
| REF 51 | Angiotensin-converting enzyme inhibition and novel cardiovascular risk biomarkers: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. Am Heart J. 2009 Feb;157(2):334.e1-8. | |||||
| REF 52 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 53 | Involvement of vascular angiotensin II-forming enzymes in the progression of aortic abdominal aneurysms in angiotensin II- infused ApoE-deficient m... J Atheroscler Thromb. 2009 Jun;16(3):164-71. | |||||
| REF 54 | Moexipril for treatment of primary biliary cirrhosis in patients with an incomplete response to ursodeoxycholic acid. Dig Dis Sci. 2010 Feb;55(2):476-83. | |||||
| REF 55 | Fixed combination perindopril-amlodipine (Coveram) in the treatment of hypertension and coronary heart disease. Rev Med Liege. 2009 Apr;64(4):223-7. | |||||
| REF 56 | An experimental model of encapsulating peritoneal sclerosis. Perit Dial Int. 2009 Feb;29 Suppl 2:S49-50. | |||||
| REF 57 | Colorimetric determination of indolic drugs. Pak J Pharm Sci. 2005 Apr;18(2):48-51. | |||||
| REF 58 | Central angiotensin II controls alcohol consumption via its AT1 receptor. FASEB J. 2005 Sep;19(11):1474-81. | |||||
| REF 59 | Selective reduction of central pulse pressure under angiotensin blockage in SHR: role of the fibronectin-alpha5beta1 integrin complex. Am J Hypertens. 2009 Jul;22(7):711-7. | |||||
| REF 60 | Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J Nat Prod. 1987 Jul-Aug;50(4):680-3. | |||||
| REF 61 | Mechanism of vasopeptidase inhibitor-induced plasma extravasation: comparison of omapatrilat and the novel neutral endopeptidase 24.11/angiotensin-converting enzyme inhibitor GW796406. J Pharmacol Exp Ther. 2005 Dec;315(3):1306-13. | |||||
| REF 62 | Radioimmunoassay for ceronapril, a new angiotensin-converting enzyme inhibitor, and its application to a pharmacokinetic study in healthy male volunteers. Ther Drug Monit. 1992 Jun;14(3):209-19. | |||||
| REF 63 | Pharmacology of idrapril: a new class of angiotensin converting enzyme inhibitors. J Cardiovasc Pharmacol. 1992 Jul;20(1):139-46. | |||||
| REF 64 | Effects of MDL 100,240, a dual inhibitor of angiotensin-converting enzyme and neutral endopeptidase on the vasopressor response to exogenous angiot... J Cardiovasc Pharmacol. 1998 Mar;31(3):408-17. | |||||
| REF 65 | Comparative effects of the dual ACE-NEP inhibitor MDL-100,240 and ramipril on hypertension and cardiovascular disease in endogenous angiotensin II-... Am J Hypertens. 2002 Feb;15(2 Pt 1):181-8. | |||||
| REF 66 | The clinical efficacy of the first Russian angiotensin-converting enzyme inhibitor methiopril in patients with heart failure. Klin Med (Mosk). 1995;73(3):49-51. | |||||
| REF 67 | Differential inhibition of plasma versus tissue ACE by utibapril: biochemical and functional evidence for inhibition of vascular ACE activity. J Cardiovasc Pharmacol. 1997 May;29(5):684-91. | |||||
| REF 68 | Enzyme immunoassays for a new angiotensin-converting enzyme inhibitor, zabicipril, and its active metabolite in human plasma: application to pharmacokinetic studies. Ther Drug Monit. 1993 Oct;15(5):448-54. | |||||
| REF 69 | Antihypertensive properties of a new long-acting angiotensin converting enzyme inhibitor in renin-dependent and independent hypertensive models. Arzneimittelforschung. 1995 Aug;45(8):853-8. | |||||
| REF 70 | Phosphinic tripeptides as dual angiotensin-converting enzyme C-domain and endothelin-converting enzyme-1 inhibitors. J Med Chem. 2010 Jan 14;53(1):208-20. | |||||
| REF 71 | Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005 Oct 20;48(21):6523-43. | |||||
| REF 72 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 73 | Antihypertensive and angiotensin converting enzyme inhibitory activities of a novel dihydrobenzofuran analogue. Arzneimittelforschung. 1988 Apr;38(4):531-6. | |||||
| REF 74 | The synthesis and angiotensin converting enzyme (ACE) inhibitory activity of chalcones and their pyrazole derivatives. Bioorg Med Chem Lett. 2010 Mar 15;20(6):1990-3. | |||||
| REF 75 | Protease inhibitors in the clinic. Med Chem. 2005 Jan;1(1):71-104. | |||||
| REF 76 | Characterization of domain-selective inhibitor binding in angiotensin-converting enzyme using a novel derivative of lisinopril. Biochem J. 2010 Apr 28;428(1):67-74. | |||||
| REF 77 | Endothelin-converting enzyme-1 inhibition and growth of human glioblastoma cells. J Med Chem. 2005 Jan 27;48(2):483-98. | |||||
| REF 78 | Moexipril, a new angiotensin-converting enzyme (ACE) inhibitor: pharmacological characterization and comparison with enalapril. J Pharmacol Exp Ther. 1995 Nov;275(2):854-63. | |||||
| REF 79 | Pharmacological and clinical profile of moexipril: a concise review.J Clin Pharmacol.2004 Aug;44(8):827-36. | |||||
| REF 80 | Angiotensin converting enzyme inhibitors. (Mercaptoaroyl)amino acids. J Med Chem. 1985 Mar;28(3):328-32. | |||||
| REF 81 | Reversal of cardiac hypertrophy and fibrosis by S21402, a dual inhibitor of neutral endopeptidase and angiotensin converting enzyme in SHRs. J Hypertens. 2000 Jun;18(6):749-55. | |||||
| REF 82 | Renal and vascular effects of S21402, a dual inhibitor of angiotensin-converting enzyme and neutral endopeptidase, in healthy subjects with hypovol... Clin Pharmacol Ther. 2002 Jun;71(6):468-78. | |||||
| REF 83 | Beneficial renal and cardiac effects of vasopeptidase inhibition with S21402 in heart failure. Hypertension. 2000 Dec;36(6):1105-11. | |||||
| REF 84 | Difluorostatine- and difluorostatone-containing peptides as potent and specific renin inhibitors. J Med Chem. 1985 Nov;28(11):1553-5. | |||||
| REF 85 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1613). | |||||
| REF 86 | New ketomethylene inhibitor analogues: synthesis and assessment of structural determinants for N-domain selective inhibition of angiotensin-converting enzyme. Biol Chem. 2012 May;393(6):485-93. | |||||
| REF 87 | Kinetic and structural characterization of amyloid-beta peptide hydrolysis by human angiotensin-1-converting enzyme. FEBS J. 2016 Mar;283(6):1060-76. | |||||
| REF 88 | Molecular Basis for Multiple Omapatrilat Binding Sites within the ACE C-Domain: Implications for Drug Design. J Med Chem. 2018 Nov 21;61(22):10141-10154. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

