Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T41141
(Former ID: TTDR01031)
|
|||||
| Target Name |
Cathepsin L (CTSL)
|
|||||
| Synonyms |
Major excreted protein; MEP; Cathepsin L1; CTSL1
Click to Show/Hide
|
|||||
| Gene Name |
CTSL
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Coagulation defect [ICD-11: 3B10] | |||||
| Function |
Important for the overall degradation of proteins in lysosomes.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.22.15
|
|||||
| Sequence |
MNPTLILAAFCLGIASATLTFDHSLEAQWTKWKAMHNRLYGMNEEGWRRAVWEKNMKMIE
LHNQEYREGKHSFTMAMNAFGDMTSEEFRQVMNGFQNRKPRKGKVFQEPLFYEAPRSVDW REKGYVTPVKNQGQCGSCWAFSATGALEGQMFRKTGRLISLSEQNLVDCSGPQGNEGCNG GLMDYAFQYVQDNGGLDSEESYPYEATEESCKYNPKYSVANDTGFVDIPKQEKALMKAVA TVGPISVAIDAGHESFLFYKEGIYFEPDCSSEDMDHGVLVVGYGFESTESDNNKYWLVKN SWGEEWGMGGYVKMAKDRRNHCGIASAASYPTV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T48K08 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 1 Clinical Trial Drugs | + | ||||
| 1 | KGP94 | Drug Info | Clinical trial | Coagulation defect | [2] | |
| Patented Agent(s) | [+] 8 Patented Agents | + | ||||
| 1 | Phenylalanine derivative 1 | Drug Info | Patented | Glioma | [3] | |
| 2 | PMID27998201-Compound-1 | Drug Info | Patented | Coronavirus infection | [3] | |
| 3 | PMID27998201-Compound-12 | Drug Info | Patented | Cancer related pain | [3] | |
| 4 | PMID27998201-Compound-17 | Drug Info | Patented | Hair loss | [3] | |
| 5 | PMID27998201-Compound-19 | Drug Info | Patented | Solid tumour/cancer | [3] | |
| 6 | PMID27998201-Compound-5 | Drug Info | Patented | Cirrhosis | [3] | |
| 7 | PMID27998201-Compound-7 | Drug Info | Patented | Abdominal aortic aneurysm | [3] | |
| 8 | PMID27998201-Compound-9 | Drug Info | Patented | Rheumatoid arthritis | [3] | |
| Preclinical Drug(s) | [+] 6 Preclinical Drugs | + | ||||
| 1 | CLIK-148 | Drug Info | Preclinical | Rheumatoid arthritis | [4] | |
| 2 | CLIK-181 | Drug Info | Preclinical | Rheumatoid arthritis | [4] | |
| 3 | L-006235-1 | Drug Info | Preclinical | Osteoarthritis | [5] | |
| 4 | SD1002 | Drug Info | Preclinical | Alzheimer disease | [4] | |
| 5 | SD1003 | Drug Info | Preclinical | Alzheimer disease | [4] | |
| 6 | Z-Phe-Ala-diazomethylketone | Drug Info | Preclinical | Alzheimer disease | [4] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 45 Inhibitor drugs | + | ||||
| 1 | KGP94 | Drug Info | [1] | |||
| 2 | Phenylalanine derivative 1 | Drug Info | [3] | |||
| 3 | PMID25399719-Compound-17 | Drug Info | [6] | |||
| 4 | PMID27998201-Compound-1 | Drug Info | [3] | |||
| 5 | PMID27998201-Compound-12 | Drug Info | [3] | |||
| 6 | PMID27998201-Compound-17 | Drug Info | [3] | |||
| 7 | PMID27998201-Compound-19 | Drug Info | [3] | |||
| 8 | PMID27998201-Compound-5 | Drug Info | [3] | |||
| 9 | PMID27998201-Compound-6 | Drug Info | [3] | |||
| 10 | PMID27998201-Compound-7 | Drug Info | [3] | |||
| 11 | PMID27998201-Compound-9 | Drug Info | [3] | |||
| 12 | CLIK-148 | Drug Info | [7] | |||
| 13 | CLIK-181 | Drug Info | [4] | |||
| 14 | L-006235-1 | Drug Info | [8] | |||
| 15 | SD1002 | Drug Info | [4] | |||
| 16 | SD1003 | Drug Info | [4] | |||
| 17 | Z-Phe-Ala-diazomethylketone | Drug Info | [4] | |||
| 18 | (3-Bromobenzoylpyridine)thiosemicarbazone | Drug Info | [1] | |||
| 19 | (S)-1-benzylcyclopentyl 1-oxohexan-2-ylcarbamate | Drug Info | [9] | |||
| 20 | (S)-tert-butyl 1-oxohexan-2-ylcarbamate | Drug Info | [9] | |||
| 21 | 1-(1,3-diphenylpropylidene)thiosemicarbazide | Drug Info | [10] | |||
| 22 | 1-(phenyl(p-tolyl)methylene)thiosemicarbazide | Drug Info | [10] | |||
| 23 | 6-(benzylamino)-9-butyl-9H-purine-2-carbonitrile | Drug Info | [11] | |||
| 24 | 9-benzyl-6-(benzylamino)-9H-purine-2-carbonitrile | Drug Info | [11] | |||
| 25 | BIPHENYL-4-YL-ACETALDEHYDE | Drug Info | [12] | |||
| 26 | Bis(3-bromophenyl)(4-hydroxy)thiosemicarbazone | Drug Info | [1] | |||
| 27 | Bis(3-bromophenyl)(5-hydroxy)thiosemicarbazone | Drug Info | [1] | |||
| 28 | Bis(3-Fluorophenyl)-ketone]thiosemicarbazone | Drug Info | [13] | |||
| 29 | CAA0225 | Drug Info | [14] | |||
| 30 | Cysteinesulfonic Acid | Drug Info | [15] | |||
| 31 | L-873724 | Drug Info | [16] | |||
| 32 | N-(4-phenylbenzoyl)-phenylalanyl-glycine-nitrile | Drug Info | [17] | |||
| 33 | N-(benzyloxycarbonyl)-leucyl-glycine-nitrile | Drug Info | [17] | |||
| 34 | N-(tert-butoxycarbonyl)-isoleucyl-glycine-nitrile | Drug Info | [17] | |||
| 35 | N-(tert-butoxycarbonyl)-leucyl-glycine-nitrile | Drug Info | [17] | |||
| 36 | N-(tert-butoxycarbonyl)-methionyl-glycine-nitrile | Drug Info | [17] | |||
| 37 | N-(tert-butoxycarbonyl)-norleucyl-glycine-nitrile | Drug Info | [17] | |||
| 38 | N-(tert-butoxycarbonyl)-tyrosyl-glycine-nitrile | Drug Info | [17] | |||
| 39 | N-(tert-butoxycarbonyl)-valyl-glycine-nitrile | Drug Info | [17] | |||
| 40 | N-acetyl-phenylalanyl-glycine-nitrile | Drug Info | [17] | |||
| 41 | N-benzoyl-phenylalanyl-glycine-nitrile | Drug Info | [17] | |||
| 42 | Peptide alpha-keto-beta-aldehyde-based inhibitors | Drug Info | [18] | |||
| 43 | [(3-Bromophenyl)-m-tolyl-ketone]thiosemicarbazone | Drug Info | [13] | |||
| 44 | [(3-Bromophenyl)-p-tolyl-ketone]thiosemicarbazone | Drug Info | [13] | |||
| 45 | [2-Phenylacetophenone]thiosemicarbazone | Drug Info | [1] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 3-Sulfinoalanine | Ligand Info | |||||
| Structure Description | Exploring inhibitor binding at the S subsites of cathepsin L | PDB:3BC3 | ||||
| Method | X-ray diffraction | Resolution | 2.20 Å | Mutation | No | [19] |
| PDB Sequence |
APRSVDWREK
10 GYVTPVKNQG20 QCGSWAFSAT31 GALEGQMFRK41 TGRLISLSEQ51 NLVDCSGPQG 61 NEGCNGGLMD71 YAFQYVQDNG81 GLDSEESYPY91 EATEESCKYN101 PKYSVANDTG 111 FVDIPKQEKA121 LMKAVATVGP131 ISVAIDAGHE141 SFLFYKEGIY151 FEPDCSSEDM 161 DHGVLVVGYG171 FESNKYWLVK186 NSWGEEWGMG196 GYVKMAKDRR206 NHCGIASAAS 216 YPTV
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: 1-Propanol | Ligand Info | |||||
| Structure Description | Structure of mutant human cathepsin L, engineered for GAG binding | PDB:6JD0 | ||||
| Method | X-ray diffraction | Resolution | 1.80 Å | Mutation | Yes | [20] |
| PDB Sequence |
TLTFDHSLEA
10 QWTKWKAMHN20 RLYGMNEEGW30 RRAVWEKNMK40 MIELHNQEYR50 EGKHSFTMAM 60 NAFGDMTSEE70 FRQVMNGFNR81 KPRKGKVFQE91 PLFYEAPRSV101 DWRKKGYVTP 111 VKNQGQCGSS121 WAFSATGALE131 GQMFRKTGRL141 ISLSEQNLVD151 CSGPQGNEGC 161 NGGYMDYAFQ171 YVQDNGGLDS181 EESYPYEATE191 ESCKYNPKYS201 VANDTGFVDI 211 PKQEKALMKA221 VATVGPISVA231 IDAGHESFLF241 YKEGIYFEPD251 CSSEDLDHAV 261 LVVGYGFEST271 ESNNKYWLVK282 NSWGEEWGNK292 GYVKMAKDRR302 NHCGIASLAS 312 YPTV
|
|||||
|
|
GLN11
3.647
LEU22
2.910
VAL34
2.167
LYS37
2.678
ASN38
1.954
MET39
3.332
MET41
3.804
ILE42
2.527
GLU43
3.144
ASN46
4.296
MET58
4.032
ALA59
2.188
MET60
1.932
ASN61
4.770
GLY64
4.844
MET66
3.164
THR67
4.291
SER68
4.269
PHE71
2.567
ARG72
3.238
GLN73
4.360
VAL74
2.207
MET75
1.846
ASN76
1.765
GLY77
3.859
LYS85
3.928
GLY86
2.139
LYS87
1.945
THR110
2.997
PRO111
2.071
VAL112
4.331
LYS113
2.030
ASN114
1.888
GLN115
2.023
CYS118
3.264
GLY119
1.850
SER120
2.378
SER121
1.566
TRP122
2.259
ALA123
4.588
PHE124
2.851
PHE135
3.651
GLY139
3.010
ARG140
2.206
LEU141
1.808
ILE142
2.944
SER143
2.280
GLU146
4.614
ASN162
4.671
GLY163
2.977
GLY164
4.708
GLN171
2.685
GLN174
2.512
GLU182
2.961
TYR187
2.871
THR206
3.311
GLY207
2.273
PHE208
2.020
VAL209
4.979
LYS213
2.643
ALA234
4.669
GLY235
2.384
HIS236
3.756
GLU237
2.295
LEU240
2.326
PHE241
3.043
SER254
2.182
GLU255
2.404
ASP258
2.664
HIS259
2.583
ALA260
3.772
TRP285
3.988
GLY286
4.942
ARG302
4.210
ASN303
1.755
GLY306
4.291
ALA308
4.815
SER309
3.090
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Putative inactive cathepsin L-like protein CTSL3P (CTSL3P) | 67.273 (74/110) | 1.68E-42 | |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
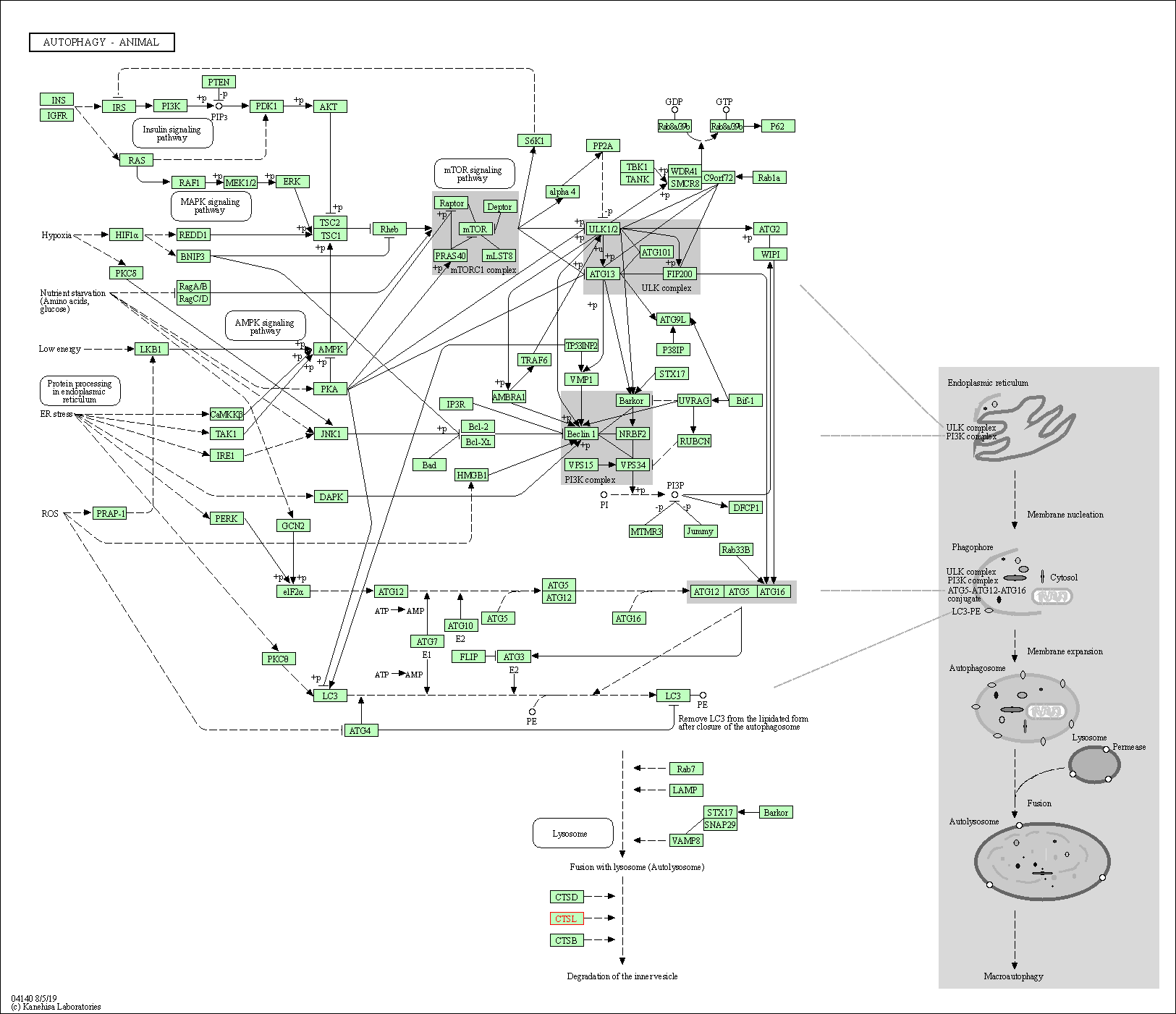
| Autophagy - animal | hsa04140 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
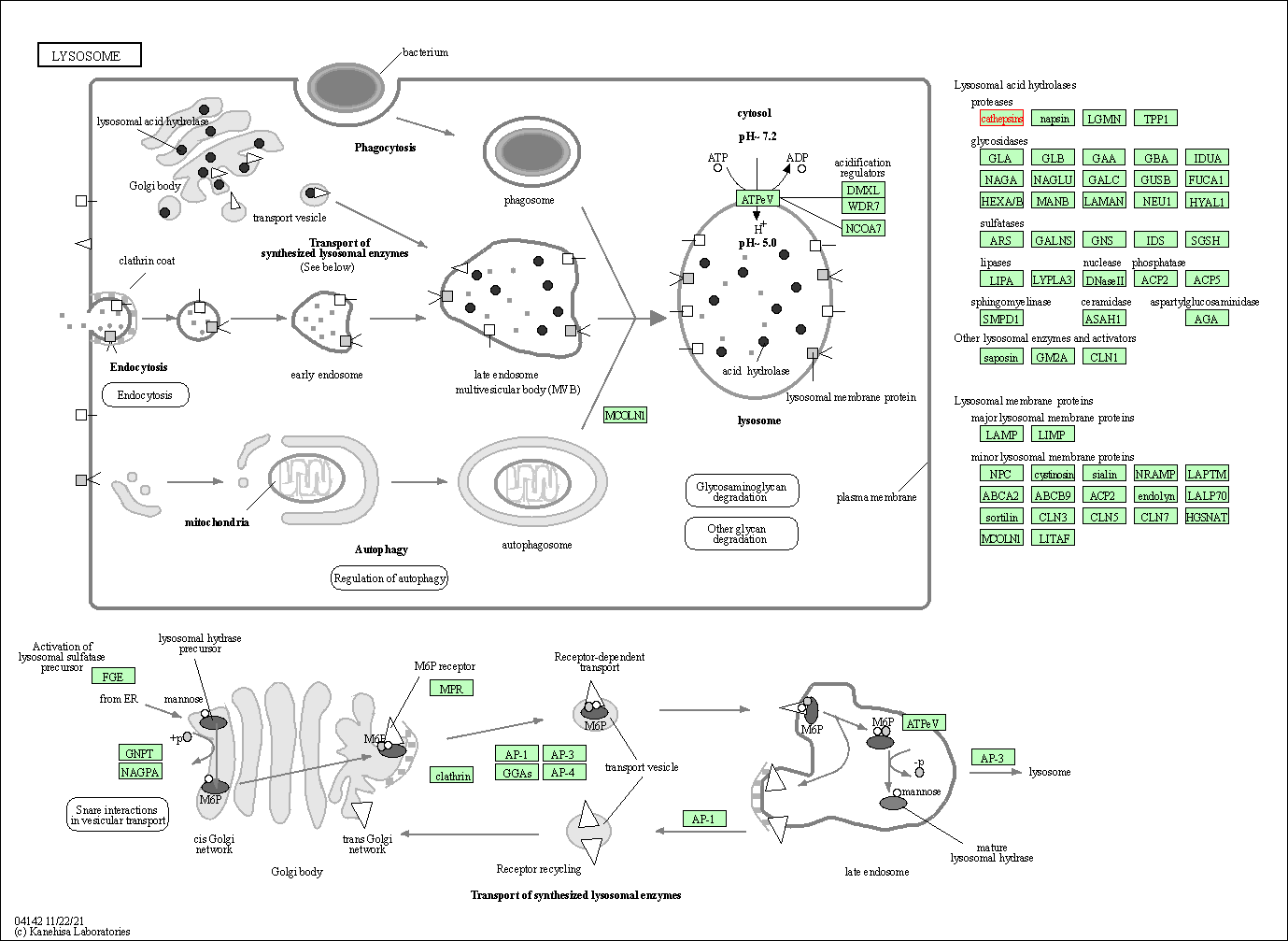
| Lysosome | hsa04142 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
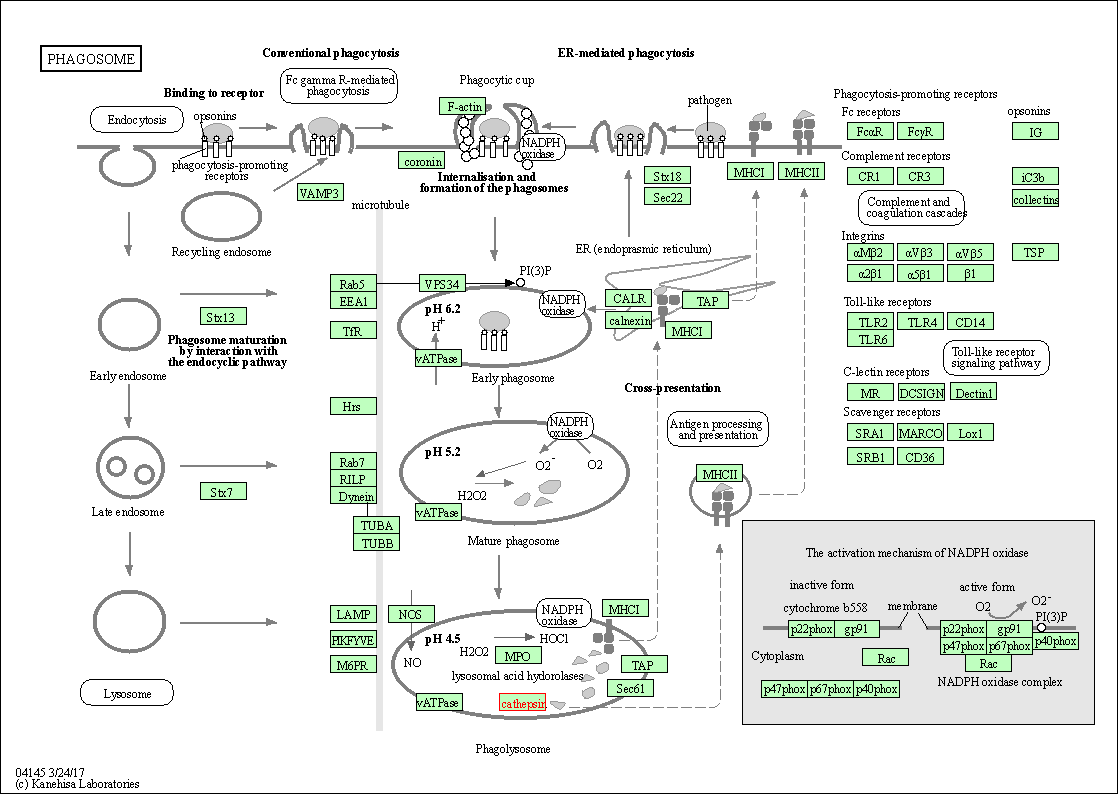
| Phagosome | hsa04145 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
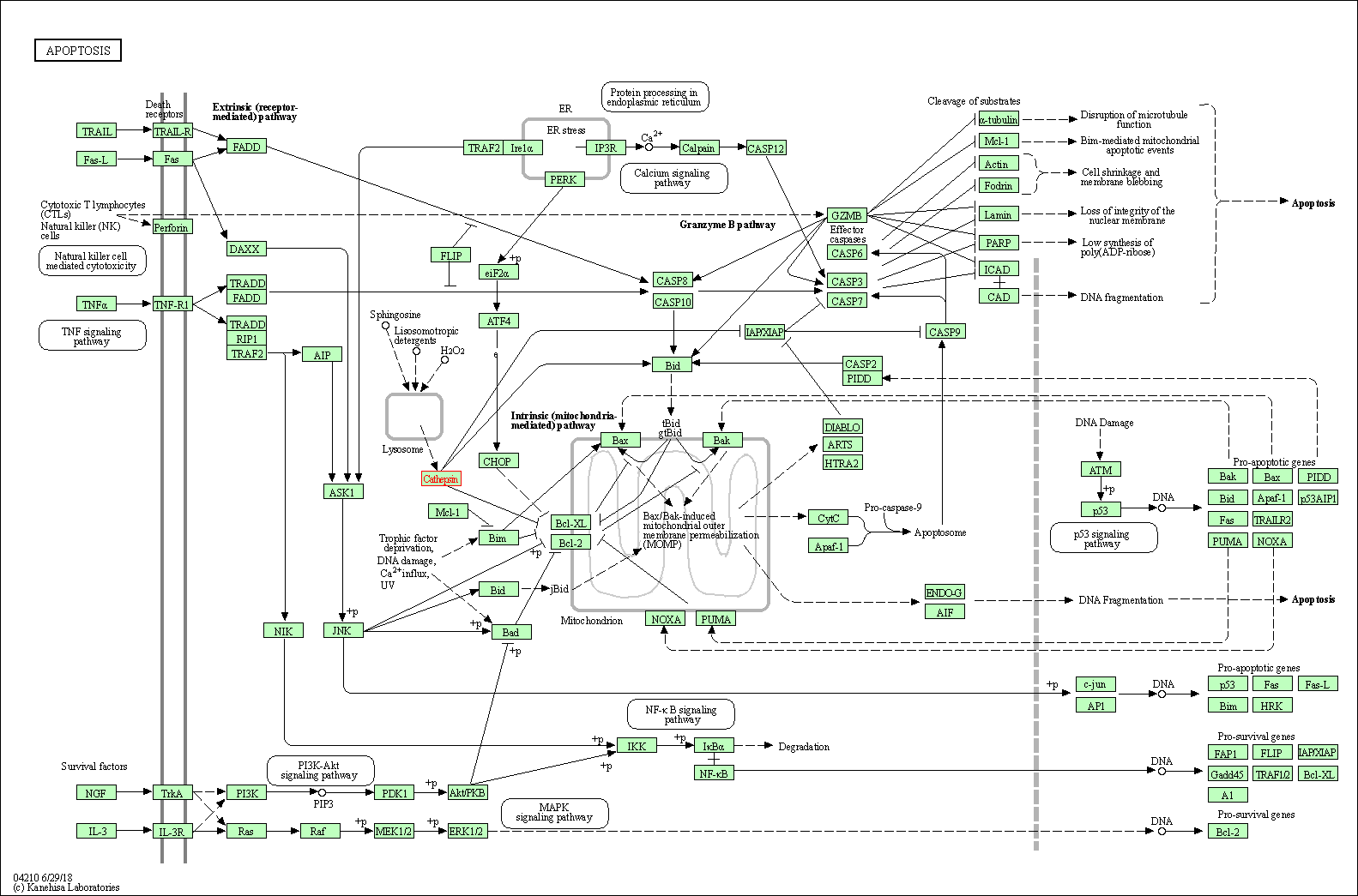
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Antigen processing and presentation | hsa04612 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 3 | Degree centrality | 3.22E-04 | Betweenness centrality | 2.36E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.70E-01 | Radiality | 1.27E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 6.33E+00 | Topological coefficient | 3.56E-01 | Eccentricity | 14 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Lysosome | |||||
| 2 | Phagosome | |||||
| 3 | Antigen processing and presentation | |||||
| 4 | Proteoglycans in cancer | |||||
| 5 | Rheumatoid arthritis | |||||
| NetPath Pathway | [+] 2 NetPath Pathways | + | ||||
| 1 | IL4 Signaling Pathway | |||||
| 2 | TGF_beta_Receptor Signaling Pathway | |||||
| Reactome | [+] 6 Reactome Pathways | + | ||||
| 1 | Endosomal/Vacuolar pathway | |||||
| 2 | Collagen degradation | |||||
| 3 | Degradation of the extracellular matrix | |||||
| 4 | Trafficking and processing of endosomal TLR | |||||
| 5 | Assembly of collagen fibrils and other multimeric structures | |||||
| 6 | MHC class II antigen presentation | |||||
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | Primary Focal Segmental Glomerulosclerosis FSGS | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Functionalized benzophenone, thiophene, pyridine, and fluorene thiosemicarbazone derivatives as inhibitors of cathepsin L. Bioorg Med Chem Lett. 2010 Nov 15;20(22):6610-5. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6533). | |||||
| REF 3 | Cathepsin B and L inhibitors: a patent review (2010 - present).Expert Opin Ther Pat. 2017 Jun;27(6):643-656. | |||||
| REF 4 | Lysosomes as a therapeutic target. Nat Rev Drug Discov. 2019 Dec;18(12):923-948. | |||||
| REF 5 | Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012 Jun;50(6):1250-9. | |||||
| REF 6 | An updated patent review of calpain inhibitors (2012 - 2014).Expert Opin Ther Pat. 2015 Jan;25(1):17-31. | |||||
| REF 7 | Structure-based development of specific inhibitors for individual cathepsins and their medical applications. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(2):29-39. | |||||
| REF 8 | Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional se... J Med Chem. 2005 Dec 1;48(24):7535-43. | |||||
| REF 9 | Semicarbazone-based inhibitors of cathepsin K, are they prodrugs for aldehyde inhibitors Bioorg Med Chem Lett. 2006 Feb 15;16(4):978-83. | |||||
| REF 10 | Discovery of trypanocidal thiosemicarbazone inhibitors of rhodesain and TbcatB. Bioorg Med Chem Lett. 2008 May 1;18(9):2883-5. | |||||
| REF 11 | Development of potent purine-derived nitrile inhibitors of the trypanosomal protease TbcatB. J Med Chem. 2008 Feb 14;51(3):545-52. | |||||
| REF 12 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 13 | Design, synthesis, and biological evaluation of potent thiosemicarbazone based cathepsin L inhibitors. Bioorg Med Chem Lett. 2010 Feb 15;20(4):1415-9. | |||||
| REF 14 | Characterization of CAA0225, a novel inhibitor specific for cathepsin L, as a probe for autophagic proteolysis. Biol Pharm Bull. 2009 Mar;32(3):475-9. | |||||
| REF 15 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 16 | The identification of potent, selective, and bioavailable cathepsin S inhibitors. Bioorg Med Chem Lett. 2007 Sep 1;17(17):4929-33. | |||||
| REF 17 | Interaction of papain-like cysteine proteases with dipeptide-derived nitriles. J Med Chem. 2005 Dec 1;48(24):7688-707. | |||||
| REF 18 | Synthesis and kinetic evaluation of peptide alpha-keto-beta-aldehyde-based inhibitors of trypsin-like serine proteases. J Pharm Pharmacol. 2001 Apr;53(4):473-80. | |||||
| REF 19 | Exploring inhibitor binding at the S' subsites of cathepsin L. J Med Chem. 2008 Mar 13;51(5):1361-8. | |||||
| REF 20 | Structure-guided protein engineering of human cathepsin L for efficient collagenolytic activity. Protein Eng Des Sel. 2021 Feb 15;34:gzab005. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

