Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T56923
(Former ID: TTDC00087)
|
|||||
| Target Name |
C-X-C chemokine receptor type 2 (CXCR2)
|
|||||
| Synonyms |
Interleukin-8 receptor B; IL8RB; IL-8R B; IL-8 receptor type 2; High affinity interleukin-8 receptor B; GRO/MGSA receptor; CXCR-2; CXC-R2; CDw128b; CD182
Click to Show/Hide
|
|||||
| Gene Name |
CXCR2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Fungal infection [ICD-11: 1F29-1F2F] | |||||
| 2 | Pain [ICD-11: MG30-MG3Z] | |||||
| Function |
Binding of IL-8 to the receptor causes activation of neutrophils. This response is mediated via a G-protein that activates a phosphatidylinositol-calcium second messenger system. Binds to IL-8 with high affinity. Also binds with high affinity to CXCL3, GRO/MGSA and NAP-2. Receptor for interleukin-8 which is a powerful neutrophil chemotactic factor.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MEDFNMESDSFEDFWKGEDLSNYSYSSTLPPFLLDAAPCEPESLEINKYFVVIIYALVFL
LSLLGNSLVMLVILYSRVGRSVTDVYLLNLALADLLFALTLPIWAASKVNGWIFGTFLCK VVSLLKEVNFYSGILLLACISVDRYLAIVHATRTLTQKRYLVKFICLSIWGLSLLLALPV LLFRRTVYSSNVSPACYEDMGNNTANWRMLLRILPQSFGFIVPLLIMLFCYGFTLRTLFK AHMGQKHRAMRVIFAVVLIFLLCWLPYNLVLLADTLMRTQVIQETCERRNHIDRALDATE ILGILHSCLNPLIYAFIGQKFRHGLLKILAIHGLISKDSLPKDSRPSFVGSSSGHTSTTL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Clotrimazole | Drug Info | Approved | Fungal infection | [2], [3] | |
| 2 | Ibuprofen | Drug Info | Approved | Pain | [4], [5] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | Reparixin | Drug Info | Phase 3 | Pancreatic islet transplantation failure | [6] | |
| 2 | AZD-5069 | Drug Info | Phase 2 | Asthma | [7] | |
| 3 | GSK1325756 | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [8], [9] | |
| 4 | PS-938285 | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [10] | |
| 5 | RIST4721 | Drug Info | Phase 2 | Palmoplantar pustulosis | [11] | |
| 6 | SB-265610 | Drug Info | Phase 2 | Asthma | [12] | |
| 7 | SB-656933 | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [13], [14] | |
| 8 | SCH-527123 | Drug Info | Phase 2 | Inflammatory bowel disease | [15] | |
| 9 | AZD-5122 | Drug Info | Phase 1 | Chronic obstructive pulmonary disease | [16] | |
| 10 | AZD4721 | Drug Info | Phase 1 | Chronic obstructive pulmonary disease | [17] | |
| 11 | SX-682 | Drug Info | Phase 1 | Melanoma | [18] | |
| Discontinued Drug(s) | [+] 2 Discontinued Drugs | + | ||||
| 1 | INDOPROFEN | Drug Info | Withdrawn from market | Gout | [19] | |
| 2 | SB-332235 | Drug Info | Discontinued in Phase 1 | Chronic obstructive pulmonary disease | [20] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | PD-157695 | Drug Info | Preclinical | Schizophrenia | [21] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Modulator | [+] 5 Modulator drugs | + | ||||
| 1 | Clotrimazole | Drug Info | [22] | |||
| 2 | Reparixin | Drug Info | [1], [23] | |||
| 3 | GSK1325756 | Drug Info | [25] | |||
| 4 | SCH-527123 | Drug Info | [30], [31] | |||
| 5 | AZD-5122 | Drug Info | [32] | |||
| Inhibitor | [+] 22 Inhibitor drugs | + | ||||
| 1 | Ibuprofen | Drug Info | [1] | |||
| 2 | AZD-5069 | Drug Info | [24] | |||
| 3 | SX-682 | Drug Info | [34] | |||
| 4 | INDOPROFEN | Drug Info | [1] | |||
| 5 | R-ketoprofen | Drug Info | [1] | |||
| 6 | (R)-2-(4-Isobutyl-phenyl)-N-methoxy-propionamide | Drug Info | [1] | |||
| 7 | (R)-2-(4-Isobutyl-phenyl)-propionamide | Drug Info | [1] | |||
| 8 | (R)-3-(4-Isobutyl-phenyl)-butan-2-one | Drug Info | [1] | |||
| 9 | (R)-N-Hydroxy-2-(4-isobutyl-phenyl)-propionamide | Drug Info | [1] | |||
| 10 | 1-(2-bromophenyl)-3-(4-cyano-2-hydroxyphenyl)urea | Drug Info | [37] | |||
| 11 | 1-(2-Hydroxy-3-nitro-phenyl)-3-phenyl-urea | Drug Info | [38] | |||
| 12 | 1-(2-hydroxy-4-nitrophenyl)-3-phenylurea | Drug Info | [37], [39] | |||
| 13 | 1-(2-Hydroxy-5-nitro-phenyl)-3-phenyl-urea | Drug Info | [38] | |||
| 14 | 1-(3-cyano-2-hydroxyphenyl)-3-phenylurea | Drug Info | [37] | |||
| 15 | 1-(4-cyano-2-hydroxyphenyl)-3-phenylurea | Drug Info | [37] | |||
| 16 | 1-(5-chloro-2-hydroxy-4-nitrophenyl)-3-phenylurea | Drug Info | [37] | |||
| 17 | 2-(3-Isobutyl-phenyl)-propionic acid | Drug Info | [1] | |||
| 18 | 2-(3-Isopropyl-phenyl)-propionic acid | Drug Info | [1] | |||
| 19 | 2-[3-(1-Hydroxy-propyl)-phenyl]-propionic acid | Drug Info | [1] | |||
| 20 | 2-[3-(1-Phenyl-ethyl)-phenyl]-propionic acid | Drug Info | [1] | |||
| 21 | 2-[3-(2-Methyl-butyl)-phenyl]-propionic acid | Drug Info | [1] | |||
| 22 | 5-(pentylthio)thiazolo[4,5-d]pyrimidin-7-ol | Drug Info | [40] | |||
| Antagonist | [+] 9 Antagonist drugs | + | ||||
| 1 | PS-938285 | Drug Info | [26] | |||
| 2 | RIST4721 | Drug Info | [27] | |||
| 3 | SB-265610 | Drug Info | [28] | |||
| 4 | SB-656933 | Drug Info | [29] | |||
| 5 | AZD4721 | Drug Info | [33] | |||
| 6 | SB-332235 | Drug Info | [28], [35] | |||
| 7 | PD-157695 | Drug Info | [36] | |||
| 8 | SB 272844 | Drug Info | [43] | |||
| 9 | SX-517 | Drug Info | [44] | |||
| Agonist | [+] 2 Agonist drugs | + | ||||
| 1 | CXCL8 | Drug Info | [41] | |||
| 2 | Il-8((3-73))K11R | Drug Info | [42] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Cholesterol | Ligand Info | |||||
| Structure Description | Cryo-EM structure of a class A GPCR monomer | PDB:6LFO | ||||
| Method | Electron microscopy | Resolution | 3.40 Å | Mutation | No | [45] |
| PDB Sequence |
SSTLPPFLLD
35 AAPCEPESLE45 INKYFVVIIY55 ALVFLLSLLG65 NSLVMLVILY75 SRVGRSVTDV 85 YLLNLALADL95 LFALTLPIWA105 ASKVNGWIFG115 TFLCKVVSLL125 KEVNFYSGIL 135 LLACISVDRY145 LAIVHATRTL155 TQKRYLVKFI165 CLSIWGLSLL175 LALPVLLFRR 185 TVYSSNVSPA195 CYEDMGNNTA205 NWRMLLRILP215 QSFGFIVPLL225 IMLFCYGFTL 235 RTLFKAHMGQ245 KHRAMRVIFA255 VVLIFLLCWL265 PYNLVLLADT275 LMRTQVIQET 285 CERRNHIDRA295 LDATEILGIL305 HSCLNPLIYA315 FIGQKFRHGL325 LKILAIH |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
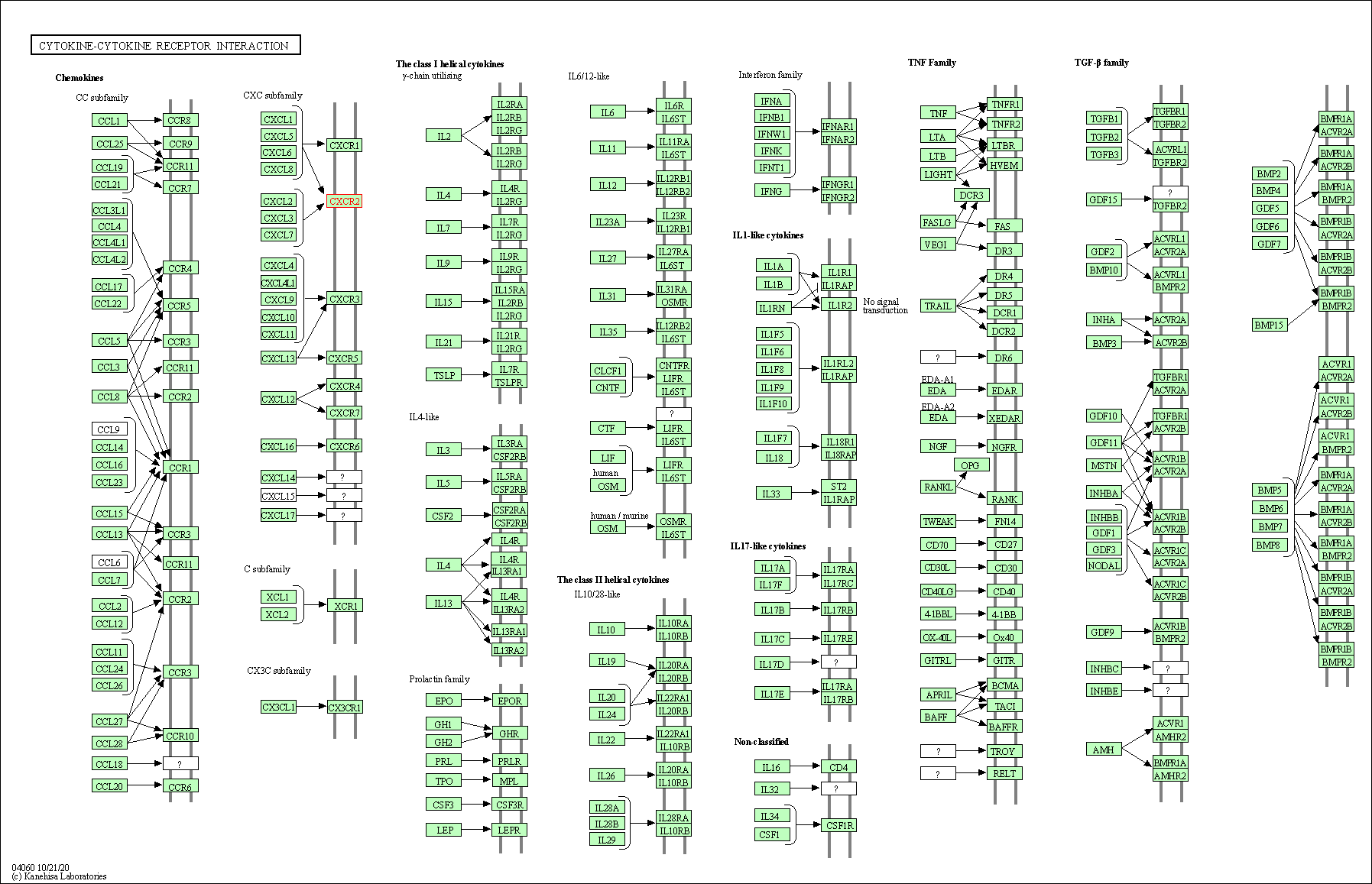
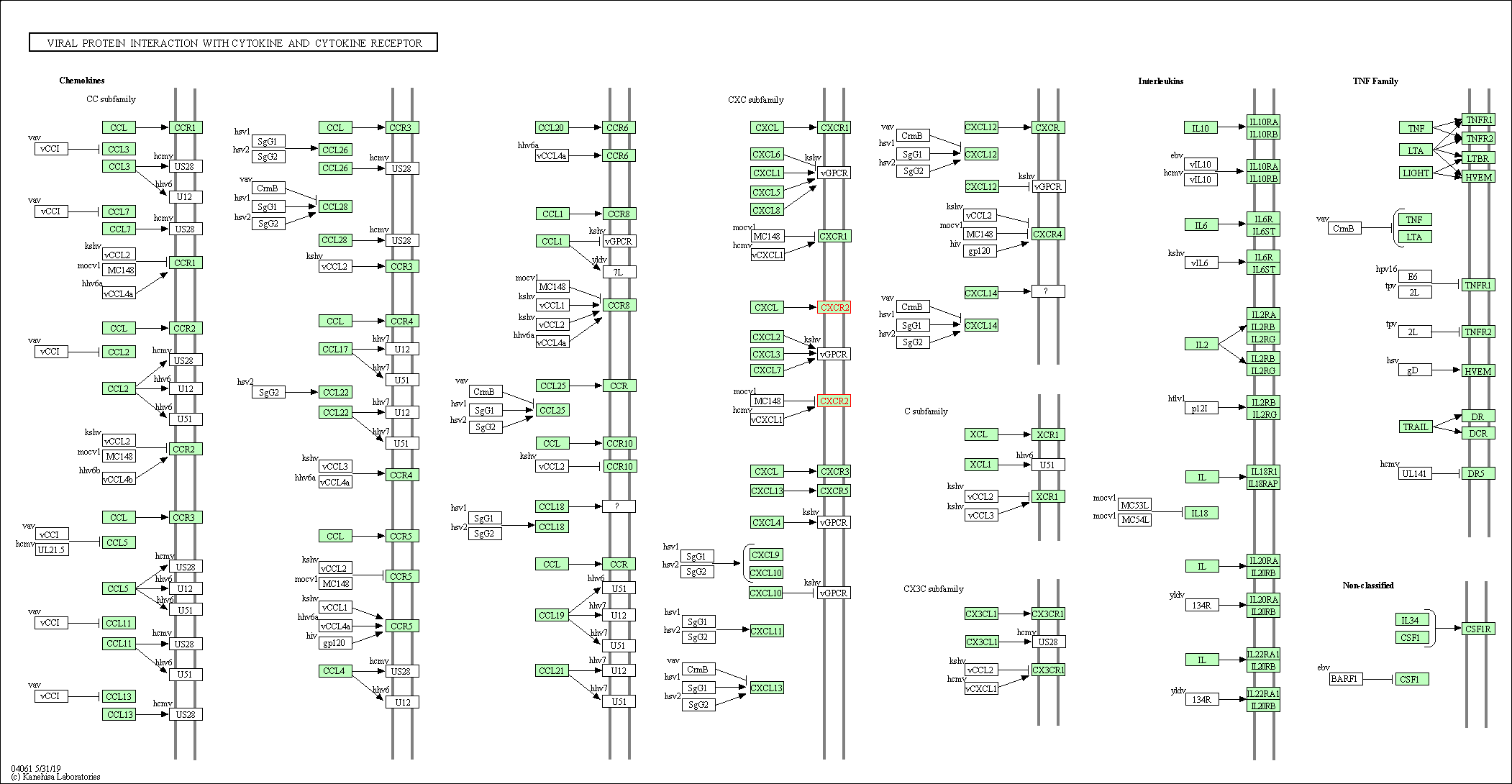

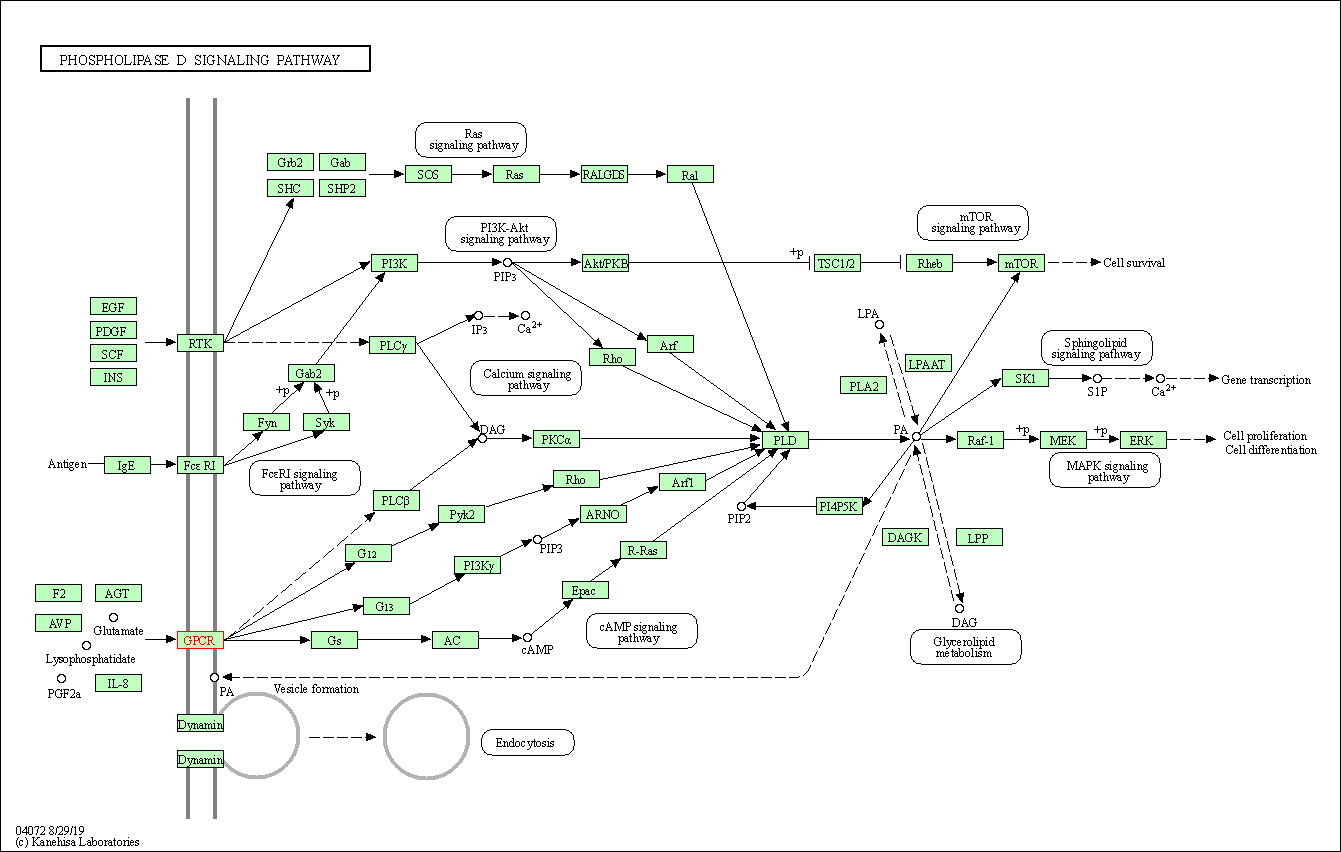
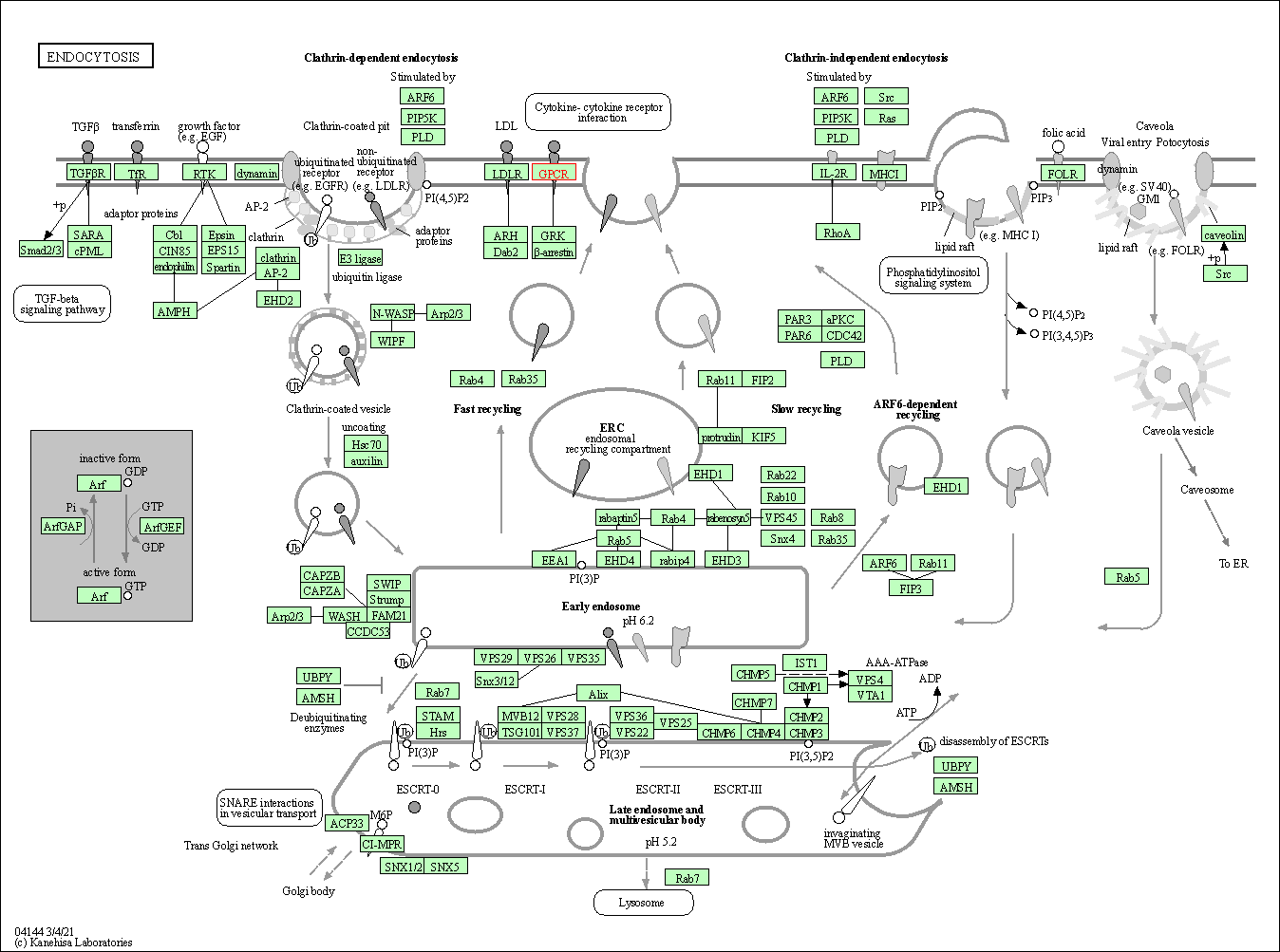
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Chemokine signaling pathway | hsa04062 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Endocytosis | hsa04144 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| Degree | 18 | Degree centrality | 1.93E-03 | Betweenness centrality | 1.38E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.21E-01 | Radiality | 1.39E+01 | Clustering coefficient | 4.58E-02 |
| Neighborhood connectivity | 1.78E+01 | Topological coefficient | 8.13E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | Chemokine signaling pathway | |||||
| 3 | Endocytosis | |||||
| 4 | Epithelial cell signaling in Helicobacter pylori infection | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TNFalpha Signaling Pathway | |||||
| Panther Pathway | [+] 2 Panther Pathways | + | ||||
| 1 | Inflammation mediated by chemokine and cytokine signaling pathway | |||||
| 2 | Interleukin signaling pathway | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | IL8- and CXCR2-mediated signaling events | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Chemokine receptors bind chemokines | |||||
| 2 | G alpha (i) signalling events | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | GPCRs, Class A Rhodopsin-like | |||||
| 2 | Peptide GPCRs | |||||
| 3 | GPCR ligand binding | |||||
| 4 | GPCR downstream signaling | |||||
| 5 | GPCRs, Other | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2-Arylpropionic CXC chemokine receptor 1 (CXCR1) ligands as novel noncompetitive CXCL8 inhibitors. J Med Chem. 2005 Jun 30;48(13):4312-31. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2330). | |||||
| REF 3 | Emerging drugs for chemotherapy-induced mucositis. Expert Opin Emerg Drugs. 2008 Sep;13(3):511-22. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2713). | |||||
| REF 5 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | |||||
| REF 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 7 | ClinicalTrials.gov (NCT01704495) A Phase II Study to Evaluate the Efficacy, Safety and Tolerability of AZD5069 in Patients With Uncontrolled Persistent Asthma. (NIMBUS). U.S. National Institutes of Health. | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8500). | |||||
| REF 9 | ClinicalTrials.gov (NCT02469298) Safety, Tolerability and Clinical Effect of Danirixin in Adults With Influenza. | |||||
| REF 10 | ClinicalTrials.gov (NCT01006161) Study of SCH 527123 in Subjects With Severe Asthma (Study P05109AM1). U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT05194839) A Randomized, Double-blind, Placebo-controlled, Dose Ranging Phase 2 Study to Evaluate the Efficacy and Safety of RIST4721 in Subjects With Palmoplantar Pustulosis. U.S.National Institutes of Health. | |||||
| REF 12 | SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol. 2011 Aug;72(2):282-93. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8499). | |||||
| REF 14 | Clinical pipeline report, company report or official report of GlaxoSmithKline. | |||||
| REF 15 | Emerging drugs to treat Crohn's disease. Expert Opin Emerg Drugs. 2007 Mar;12(1):49-59. | |||||
| REF 16 | ClinicalTrials.gov (NCT00984477) Study to Investigate the Activity of AZD5122 When Given as a Single Dose to Healthy Male Subjects. U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT01889160) Study to Investigate the Safety Profile of AZD4721 After Single Doses at Different Dose Levels. U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT03161431) SX-682 Treatment in Subjects With Metastatic Melanoma Concurrently Treated With Pembrolizumab. U.S. National Institutes of Health. | |||||
| REF 19 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014165) | |||||
| REF 21 | The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007 Oct;12(10):904-22. | |||||
| REF 22 | Mode of action of clotrimazole: implications for therapy. Am J Obstet Gynecol. 1985 Aug 1;152(7 Pt 2):939-44. | |||||
| REF 23 | Reparixin, an inhibitor of CXCR1 and CXCR2 receptor activation, attenuates blood pressure and hypertension-related mediators expression in spontane... Biol Pharm Bull. 2011;34(1):120-7. | |||||
| REF 24 | Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther. 2015 May;353(2):340-50. | |||||
| REF 25 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 26 | CenterWatch. Drugs in Clinical Trials Database. CenterWatch. 2008. | |||||
| REF 27 | A Randomized, Double-Blind, Placebo-Controlled, Phase 2a Study to Evaluate the Efficacy and Safety of RIST4721 in Subjects with Palmoplantar Pustulosis. Dermatol Ther (Heidelb). 2021 Dec;11(6):2179-2193. | |||||
| REF 28 | Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2006 May;11(2):275-91. | |||||
| REF 29 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 30 | CXCR2 Antagonist MK-7123.A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med.2015 May 1;191(9):1001-11. | |||||
| REF 31 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 32 | Molecular Approaches To Target GPCRs in Cancer Therapy. Pharmaceuticals 2011, 4(4), 567-589. | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038326) | |||||
| REF 34 | Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res. 2020 Mar 15;26(6):1420-1431. | |||||
| REF 35 | A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J Immunol. 2002 Dec 1;169(11):6435-44. | |||||
| REF 36 | Interleukin-8 receptor antagonists in pulmonary diseases. Curr Opin Pharmacol. 2001 Jun;1(3):242-7. | |||||
| REF 37 | Comparison of N,N'-diarylsquaramides and N,N'-diarylureas as antagonists of the CXCR2 chemokine receptor. Bioorg Med Chem Lett. 2007 Mar 15;17(6):1713-7. | |||||
| REF 38 | Evaluation of potent and selective small-molecule antagonists for the CXCR2 chemokine receptor. J Med Chem. 2004 Mar 11;47(6):1319-21. | |||||
| REF 39 | Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998 Apr 24;273(17):10095-8. | |||||
| REF 40 | Hit-to-Lead studies: the discovery of potent, orally bioavailable thiazolopyrimidine CXCR2 receptor antagonists. Bioorg Med Chem Lett. 2006 Feb 15;16(4):960-3. | |||||
| REF 41 | Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11791-6. | |||||
| REF 42 | Il-8((3-73))K11R is a high affinity agonist of the neutrophil CXCR1 and CXCR2. Biochem Biophys Res Commun. 2001 Aug 24;286(3):595-600. | |||||
| REF 43 | The selective CXCR2 antagonist SB272844 blocks interleukin-8 and growth-related oncogene-alpha-mediated inhibition of spontaneous neutrophil apoptosis. Pulm Pharmacol Ther. 2002;15(2):103-10. | |||||
| REF 44 | Discovery of 2-[5-(4-Fluorophenylcarbamoyl)pyridin-2-ylsulfanylmethyl]phenylboronic Acid (SX-517): Noncompetitive Boronic Acid Antagonist of CXCR1 and CXCR2. J Med Chem. 2014 Oct 23;57(20):8378-97. | |||||
| REF 45 | Structural basis of CXC chemokine receptor 2 activation and signalling. Nature. 2020 Sep;585(7823):135-140. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

